Toyobo signs an agreement with U.S. distributor to expand Nerbridge® sales
Toyobo Co., Ltd. has signed an exclusive U.S. agreement with Synovis Micro Companies Alliance Inc. (hereafter Synovis MCA), a subsidiary of Baxter International Inc., as distributor for Nerbridge®, a conduit for regenerating damaged peripheral nerves. Synovis MCA plans to start selling the product in the United States in September 2018.
1. Background
In 2013, Toyobo began domestic sales of Nerbridge® as Japan’s first medical device to facilitate regeneration of peripheral nerves severed or damaged due to external injury. Nerbridge® differs from existing treatment methods, because it allows treatment without the harvesting of healthy nerves taken from the patient for autografting. Accordingly, it is considered to be an effective and safe nerve regeneration device that reduces the physical burden on the patient. Since its market debut, Nerbridge® has been used at medical institutions across Japan.
2. How the agreement with Synovis MCA was reached
Synovis MCA’s initial entry into the nerve market was in 2005 with the nerve conduit named “Neurotube”. The company boasts outstanding sales records on the strength of its robust sales networks and extensive networks with the medical care sector. Toyobo concluded a distributor agreement with Synovis MCA because Nerbridge®’s unique advantages and reliability backed up by its sales record in Japan matches the needs in the U.S., which is the world’s largest market of such nerve regeneration devices.
3. Business schedule
After Nerbridge® is introduced at the American Society for Surgery of the Hand meeting to be held in Boston, September 13-15, Synovis MCA will start limited sales of the product as a precursor to full-fledged sales in 2019. Furthermore, Toyobo plans to expand its sales outside the United States in 2020.
Main features of Nerbridge®
Nerbridge® is a tube made of polyglycolic acid filled with collagen to facilitate nerve growth, which differentiates it from other products currently available in the U.S. By inserting and fixing Nerbridge® between the ends of a severed nerve, the product induces regeneration and extension of the nerve from the central nervous system side into the peripheral nerve through the tube, restoring the nerve functions. Using Nerbridge® makes it unnecessary to harvest healthy peripheral nerves for autografting, considerably reducing the patient’s burden and shortening the time needed for surgical treatment. The polyglycolic acid and collagen used in Nerbridge® dissolve and are absorbed by the patient’s body after the nerve is regenerated.

Nerbridge®
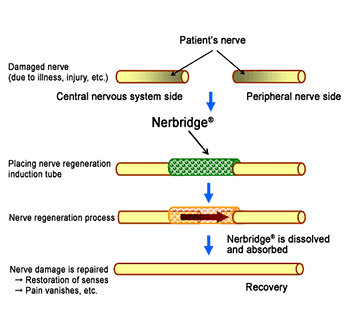
Process of treating damaged nerves with Nerbridge®
Outline of the two U.S. firms
Synovis Micro Companies Alliance Inc.
| Headquarters | : | 439 Industrial Lane, Birmingham, AL 35211, USA |
|---|---|---|
| President | : | Michael K. Campbell |
| Main business operations | : | Sales of devices for precision surgeries |
Baxter International Inc.
| Headquarters | : | 1 Baxter Parkway, Deerfield, IL 60015, USA |
|---|---|---|
| Chairman, President and CEO | : | José E. Almeida |
| Main business operations | : | Development, manufacturing and sales of drugs and medical equipment to treat immunodeficiency, cancers, infectious diseases, kidney diseases and external injuries |
For more information, contact
Public Relations Group, Corporate Communication Department, Toyobo Co., Ltd.
MAILpr_g@toyobo.jp
Cautionary Statement
This website contains forward-looking statements that reflect Toyobo's plans and expectations. These forward-looking statements are not guarantees of future performance and involve known and unknown risks, uncertainties and other factors that may cause Toyobo's actual results, performance, achievements or financial position to be materially different from any future results, performance, achievements or financial position expressed or implied by these forward-looking statements.




