-
Synthetic coating material
「SEC ONE SURFACETM」
Outline
SEC ONE SURFACETM is a biocompatible polymer, which can introduce antithrombogenicity on medical devices that contact human organs or vessels.
-
-
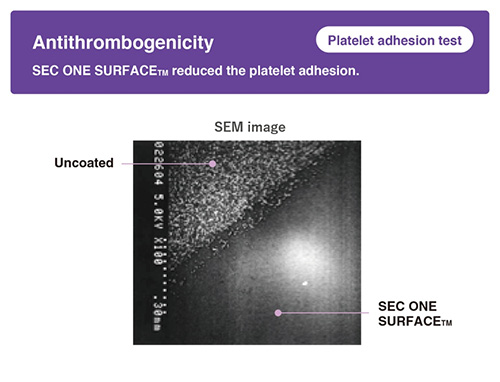
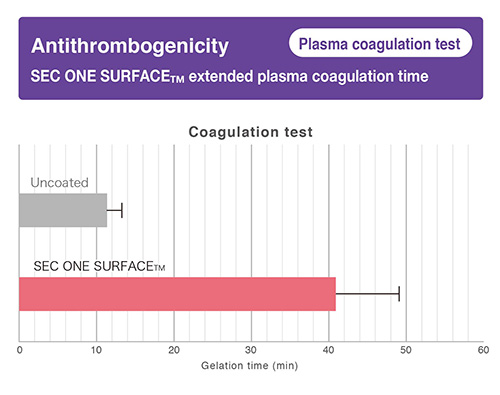
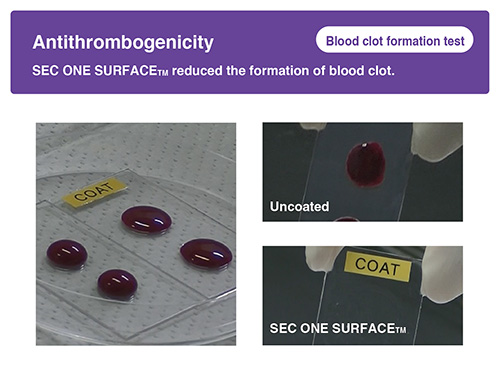
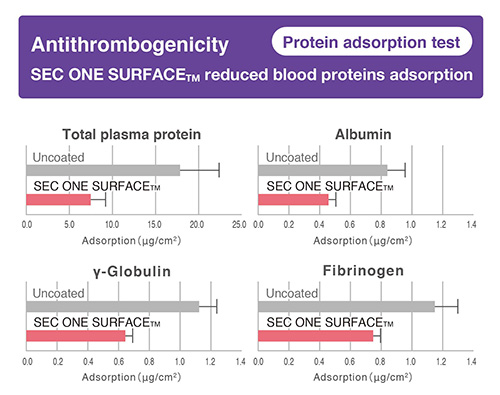
- 【Antithrombogenicity】
- Expected a reduction of thrombosis by suppressing “platelet adhesion”, “protein adsorption” and “plasma coagulation”.
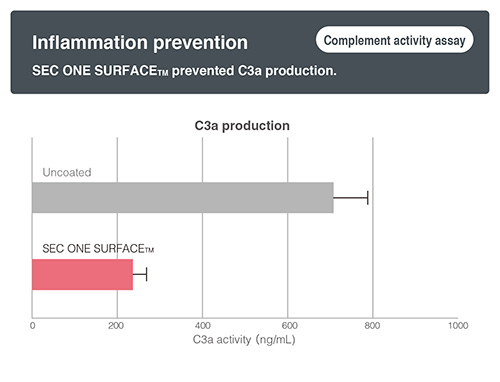
- 【Inflammation prevention】
- Expected a suppression of inflammation reaction by preventing “complement activation”.
- 【Base material affinity】
- Has adhesion and consistent coating thickness on base materials, can be applied for various medical device components.
- 【Biologic free】
- Is a synthetic polymer and can eliminates potential virus contamination with biologics.
- 【Easiness】
- Can be coated by dissolving the polymer in solvents, contacting the substrates and drying.
-
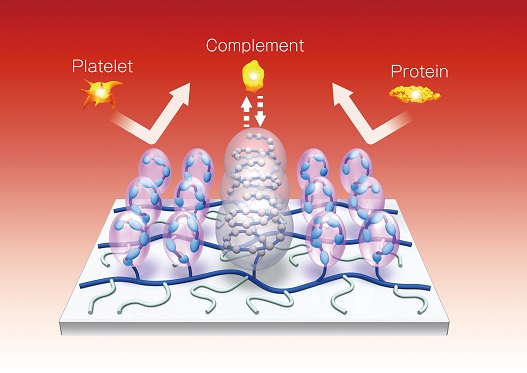
Three units on SEC ONE SURFACETM contribute to its biocompatibility.
When medical device surface contacts blood, three systems, complement, platelet and coagulation systems are activated by foreign body recognition. After the complement system activation, C3a is generated and leads to inflammation.
After platelet system and coagulation systems activations, they lead to blood clot by platelet adhesion and plasma coagulation.
The 3 units on SEC ONE SURFACETM can prevent foreign body recognitions when contact with blood.

-
- 【Steps to coat the polymer on base materials】
- ・Step1:Dissolve the polymer into suitable solvents for base materials and make a solution.
- ・Step2:Dip or spray the solution to coat on the base materials.
- ・Step3:Remove the solvents by drying.
Feature
Antithrombogenicity
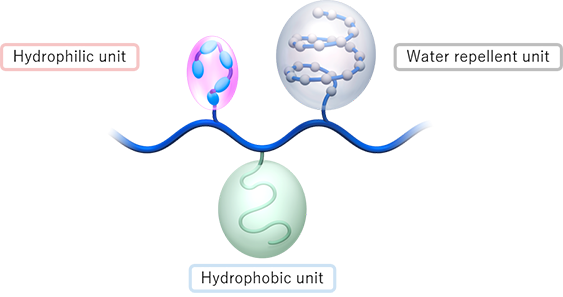
Hydrophilic unit reduces platelet adhesion, blood clot formation and microbes attachment.
Inflammation prevention
Water repellent unit reduces complement activation.
Base material affinity
Hydrophobic unit enhances the adhesiveness and consistent coating thickness on base materials and reduces the polymer elution.
- ・Plasticized Polyvinyl Chloride
- ・Nylon
- ・ABS resin
- ・Polycarbonate
- ・Polyurethane
- ・Polypropylene
- ・Polyethylene
- ・Ethylene-Vinyl Acetate Copolymer
- ・Glass
- ・Stainless Steel
- ・Aluminum
- ・Fluorinated resins including Polytetrafluoroethylene
- ・Silicone Resin
Information
Specifications
| Appearance | Transparent viscous liquid |
|---|---|
| Polymer design | ・Copolymer with Polyethylene glycol acrylate, Alkyl acrylate and Polydimethyl siloxane methacrylate ・Water insoluble |
| Water contact angle | 40-60° |
| Applicable sterilization method | Ethylene oxide sterilization |
Biocompatibility testing
| Testing | Method* | Result |
|---|---|---|
| Cytotoxicity | ISO10993-5*, ISO10993-5, ② | Negative |
| Sensitization | ISO10993-10*, ISO10993-10, ① | Negative |
| Acute systemic toxicity | ISO10993-11*, ISO10993-11, ① | Negative |
| Pyrogenicity | ISO10993-11*, ISO10993-11, ① | Negative |
| Hemolysis | ISO10993-4*, ISO10993-4, ① | Negative |
| Intracutaneous injection | ISO10993-10*, ISO10993-10, ① | Negative |
| Reverse mutation | ISO10993-3 | Negative |
| Chromosomal aberration | ISO10993-3 | Negative |
| Implantation for 2 and 4 weeks | ISO10993-6 | Negative |
| Subchronic toxicity for 28-day continuous venous administration |
ISO10993-11 | Negative |
① “Guidance for Specific Biological Tests Relevant to the Principles Issued by the Notification Iyakushinhatsu No. 0213001”, Pharmaceutical and Food Safety Bureau, Japanese Ministry of Health, Labour and Welfare, Memorandum Medical Device Review No.36, March 19, 2003
② “Revision of the Basic Principles of Biological Safety Assessment Required for Medical Device Manufacturing and Sales Approval Applications(No.1, Yakuseikishinhatsu 0106)”issued by Medical Device Evaluation and Management Division, Pharmaceutical and Food Safety Bureau, Japanese Ministry of Health, Labour and Welfare, January 6, 2020
※ Tested according to “Good Laboratory Practice Standard Ordinance for Nonclinical Laboratory Studies on Safety of Medical Devices”, Japanese Ministry of Health, Labour and Welfare, Ordinance No. 37, March 23, 2005
-
CONTACT
-
For inquiries or consultations regarding our products, please contact us.