- HOME
- Enzyme List
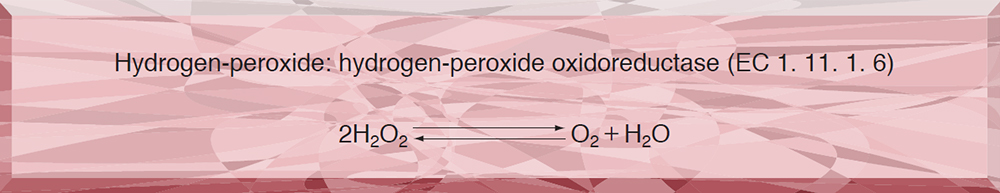
- CAO-519 CATALASE
CAO-519
CATALASE from Microorganism
PREPARATION and SPECIFICATION
| Appearance | Olive green solution |
|---|---|
| Activity | GradeⅤ 150,000 U/mL or more |
PROPERTIES
| Stability | Stable at 4 ℃(Fig.1) |
|---|---|
| Molecular weight | approx. 53,000 (by mass spectrometry) |
| Inhibitors | NaN3 |
| Optimum pH | 7.2-9.0(Fig.2) |
| Optimum temperature | 35-40 ℃(Fig.3) |
| pH Stability | pH 6.2-8.9 (25 ℃, 16 hr)(Fig.4) |
| Thermal stability | below 35 ℃ (pH 7.0, 30 min)(Fig.5) |
| Effect of various chemicals | (Table 1) |
APPLICATIONS
This enzyme is useful for eliminating the interference of hydrogen peroxide in clinical analysis.
ASSAY
Principle
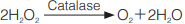
The elimination of hydrogen peroxide is measured by the titanium color method 1).
Unit definition
One unit causes the hydrolysis of one micromole of hydrogen peroxide per minute under the conditions detailed below.
Method
Reagents
| A. 10 mM Phosphate buffer, pH 7.0 (at 25 ℃) | |
|---|---|
| B. H2O2 solution | 16 mM[0.182 mL of 30 % (W/V) H2O2/100 mL of buffer A](Should be prepared fresh and stored ice.) |
| C. Titanium reagent | (Nacalai Tesque) |
| D. Enzyme diluent | Buffer A |
Procedure
1.Prepare 0.25 mL of the substrate solution (B) in a test tube and equilibrate at 25 ℃ for about 5 minutes
2.Add 0.25 mL of the enzyme solution* and mix.
3.After exactly 5 minutes at 25 ℃, add 2.5 mL of titanium reagent (C) to stop the reaction and measure the optical density at 410 nm against water (OD test)
At the same time, prepare the blank by mixing the substrate solution with 2.5 mL of titanium reagent after incubation for 5 minutes at 25 ℃, followed by the addition of enzyme solution (OD blank)
*Dilute the enzyme preparation to 0.35-1.35 U/mL with ice-cold enzyme diluent (D).
Calculation
Activity can be calculated by using the following formula :
Volume activity (U/mL) =
-
ΔOD(OD blank-OD test)×Vt×df
F×t×1.0×Vs
= ΔOD/min×2.4×1/F×df
Weight activity (U/mg) = (U/mL)×1/C
| Vt | : Total volume (3.0 mL) |
| Vs | : Sample volume (0.25 mL) |
| F | : Extinction coefficient of Titanium color product developed by the presence of 1.0 mM hydrogen peroxide (F should be determined in each lot of Titanium reagent by using a known concentration of hydrogen peroxide. F is usually around 0.7.) |
| t | : Reaction time (5 minutes) |
| 1.0 | : Light path length (cm) |
| df | : Dilution factor |
| C | : Enzyme concentration in dissolution (c mg/mL) |
REFERENCES
1)F.Patti and P.B.-Maury; Bull.Soc.Chem.Biol.,35, 1177 (1953)
Table 1. Effect of Various Chemicals on Catalase
[The enzyme dissolved in 10 mM K-phosphate buffer, pH 7.0 (200 U/mL) was incubated with each chemical at 25 ℃ for 1 hr.]
-
Chemical Concn.(mM) Residual activity(%) None - 100 Metal salt 2.0 AgNO3 76.6 BaCl2 99.6 CaCl2 62.4 CoCl2 101.2 CuSO4 99.7 FeSO4 98.2 MgSO4 99.2 MnCl2 28.7 NiCl2 53.6 ZnCl2 96.1 -
Chemical Concn.(mM) Residual activity(%) NaF 2.0 89.3 NaN3 2.0 3.8 EDTA 5.0 96.6 IAA 2.0 99.4 Borate 20 98.8 SDS 0.05 % 96.1 Triton X-100 0.10 % 95.7 Brij 35 0.10 % 97.8 Span 20 0.10 % 94.5 Na-cholate 0.10 % 95.3
DAC, Dimethylbenzylalkylammonium chloride
-
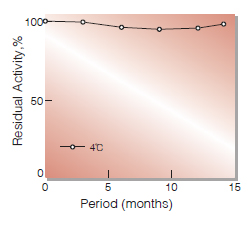
Fig.1. Stability (Liquid form)
-
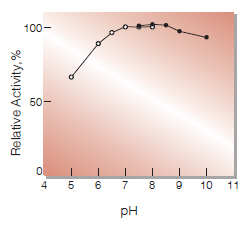
Fig.2. pH-Activity
in 10 mM buffer solution: pH 5-8,K-phosphate; pH 7.5-10, Tris-HCl
-
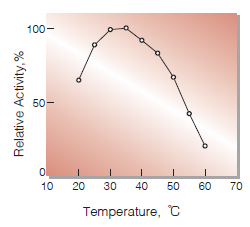
Fig.3. Temperature activity
(in 10 mM K-phosphate buffer, pH 7.0)
-
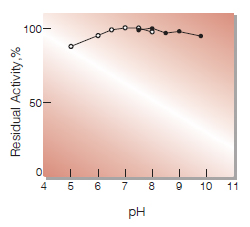
Fig.4. pH-Stability
in 10 mM buffer solution: pH 5-8,K-phosphate; pH 7.5-10, Tris-HCl.Enzyme concentration: 1 U/mL
-
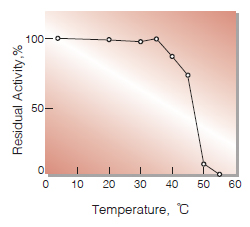
Fig.5. Thermal stability
30 min-treatment with 10 mM K-phosphate buffer, pH 7.0.Enzyme concentration: 200 U/mL
活性測定法(Japanese)
1. 原理
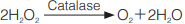
過酸化水素の減少量をチタン呈色法で測定する。
2.定義
下記条件下で1分間に1マイクロモルの過酸化水素を分解する酵素量を1単位(U)とする。
3.試薬
- 10mM リン酸緩衝液, pH 7.0
- H2O2溶液:16mM[0.182 mLの30%(W/V)H2O2を100 mLのbuffer Aに溶解する](用時調製し,使用時は氷冷保存する)
- チタン試薬:(ナカライテスク製)
- 酵素溶液:酵素備品を予め氷冷した緩衝液Aで0.35~1.35U/mLに希釈する。
4.手順
1.試験管に0.25 mLの基質溶液(B)を採り,25℃で約5分間予備加温する。
2.酵素溶液0.25 mLを添加し,緩やかに混和する。
3.25℃で正確に5分間反応させた後,チタン試薬(C)2.5 mLを加えて反応を停止させ,水を対照にして410nmの吸光度を測定する(OD test)。
4.盲検は5分間の反応の後, 最初に基質溶液(B)0.25㎖をチタン試薬(C)2.5 mLに加えて混和し,次いで酵素溶液を添加する(OD blank)。
5.計算式
U/mL =
-
ΔOD (OD blank-OD test)×3.0(mL)×希釈倍率
F×5(分)×1.0×0.25(mL)
| = ΔOD/min×2.4×1/F×df | |
| U/mg | = U/mL×1 / C |
| F | 1.0mM過酸化水素によるチタン呈色生成物の吸光係数 (Fは濃度の分かっている過酸化水素を用いて各ロット毎に決定する。通常は0.7前後である) |
| 1.0 | 光路長(cm) |
| C | 溶解時の酵素濃度(c mg/mL) |
CONTACT
-
For inquiries and cosultations regarding our products, please contact us through this number.
- HEAD OFFICE+81-6-6348-3843
- Inquiry / Opinion
