- HOME
- Enzyme List
- CRH-211 CREATINE AMIDINOHYDROLASE
CRH-211
CREATINE AMIDINOHYDROLASE from Microorganism
PREPARATION and SPECIFICATION
| Appearance | White amorphous powder, lyophilized | |
|---|---|---|
| Activity | GradeⅡ 6.0 U/mg-solid or more (containing approx. 50 % of stabilizers) |
|
| Contaminants | NADH oxidase | ≤ 5.0×10-2 % |
| Catalase | ≤ 2.0 % | |
| Stabilizers | Sugars, EDTA | |
PROPERTIES
| Stability | Stable at −20 ℃ for at least one year(Fig.1,2) |
|---|---|
| Molecular weight | approx. 100,000 |
| Isoelectric point | 4.6±0.1 |
| Michaelis constant | 1.9×10-2 M (Creatine) |
| Structure | 2 subunits per enzyme molecule |
| Inhibitors | Cu2+,Hg2+,Ag+ |
| Optimum pH | 8.0(Fig.4) |
| Optimum temperature | 40 ℃(Fig.5) |
| pH Stability | pH 5.5−9.0 (25 ℃, 16 hr)(Fig.6) |
| Thermal stability | below 50 ℃ (pH 7.5, 30 min)(Fig.7) |
| Effect of various chemicals | (Table 1) |
APPLICATIONS
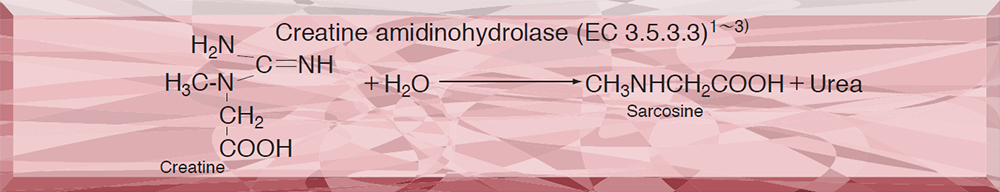
This enzyme is useful for enzymatic determination of creatine and creatinine in combination with creatinine amidohydrolase (CNH-211, CNH-311) and sarcosine oxidase (SAO-351) in clinical analysis.4)
ASSAY
Principle

The formation of yellow dye by condensation of urea and p-dimethylaminobenzaldehyde (DAB) in the Ehrlich reaction is measured at 435 nm by spectrophotometry.
Unit definition
One unit causes the formation of one micromole of yellow dye per minute under the conditions detailed below.
Method
Reagents
| A. Creatine solution | 0.1 M [1.49 g creatine (Nacalai tesque)/100 mL of 50 mM phosphate buffer, pH 7.5](Should be prepared fresh). |
|---|---|
| B. DAB solution | Dissolve 2.0 g of DAB in 100 mL of dimethylsulfoxide and, to this solution, add 15 mL of conc. HCl solution. |
| C. Enzyme diluent | 50 mM Phosphate buffer, pH 7.5 |
Procedure
1.Pipette 1.0 mL of the substrate solution (A) into a test tube and equilibrate at 37 ℃ for approximately 5 minutes.
| Concentration in assay mixture | |
|---|---|
| Phosphate buffer | 50 mM |
| Creatine | 90 mM |
2.Add 0.1 mL of the enzyme solution* and mix.
3.After exactly 10 minutes at 37 ℃, add 2.0 mL of DAB solution (B) to stop the reaction.
4.Incubate at 25 ℃ for 20 minutes.
5.Measure the optical density at 435nm against water (OD test).
At the same time, prepare the OD blank by mixing the substrate solution with 2.0 mL of DAB solution after incubation for 10 minutes at 37℃, followed by addition of the enzyme solution, and then perform the same procedures as in the test (procedure 4 and 5).
*Dissolve the enzyme preparation in ice-cold enzyme diluent (C) and dilute to 2.0-3.0 U/mL with the same buffer, immediately before the assay.
Calculation
Activity can be calculated by using the following formula :
Volume activity (U/ml) =
-
ΔOD (OD test−OD blank)×Vt×df
0.321×1.0×t×Vs
= ΔOD×9.65×df
Weight activity (U/mg) = (U/mL)×1/C
| Vt | : Total volume (3.1 mL) |
| Vs | : Sample volume (0.1 mL) |
| 0.321 | : Millimolar extinction coefficient of yellow dye(cm2/micromole) |
| 1.0 | : Light path length (cm) |
| t | : Reaction time (10 minutes) |
| df | : Dilution factor |
| C | : Enzyme concentration in dissolution (c mg/mL) |
REFERENCES
1)D.Tsuru; Nucleic Acid and Amino Acids, 35, 31 (1977).
2)T.Yoshimoto, I.Oka and D.Tsuru; Arch.Biochem.Biophys., 177, 508 (1976).
3)T.Yoshimoto ,I.Oka and D.Tsuru; J.Biochem., 79, 1381 (1976).
4)D.Tsuru; Rinsho Kensa, 22, 1331 (1978).
Table 1. Effect of Various Chemicals on Creatine amidinohydrolase
[The enzyme dissolved in 50mM Tris-HCl buffer, pH 7.5 (80U/mL) was incubated at 25℃ for 30 minutes with each chemical.]
-
Chemical Concn.(mM) Residual activity(%) None ー 100 Metal salt 1.0 CaCl2 107 MnCl2 109 MgCl2 103 NiCl2 107 CoCl2 108 Ba(OAc)2 104 Cd(OAc)2 88 FeCl3 103 FeSO4 102 HgCl2 2.7 ZnSO4 97 CuSO4 11 Pb(OAc)2 108 AgNO3 2.5 EDTA 20 99 α,α′-Dipyridyl 1.0 100 -
Chemical Concn.(mM) Residual activity(%) NaF 1.0 105 PCMB 0.33 3.3 MIA 1.0 106 IAA 1.0 103 NaN3 10 106 o-Phenanthroline 1.0 108 Hydroxylamine 1.0 105 NEM 10 0.3 Triton X-100 0.5 % 94 Brij 35 0.5 % 103 Tween 20 0.5 % 100 Span 20 0.5 % 106 Na-cholate 0.5 % 103 SDS 0.25 % 102 DAC 0.5 % 1.7
IAA, lodoacetamide; NEM, N-Ethylmaleimide; SDS, Sodium dodecyl sulfate; DAC, Dimethylbenzylalkylammonium chloride.
-
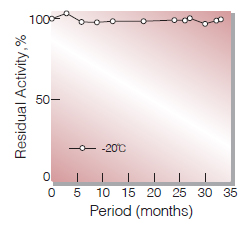
Fig.1. Stability (Powder form)
(kept under dry conditions)
-
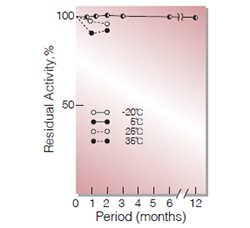
Fig.2. Stability (Powder form)
(kept under dry conditions)
-
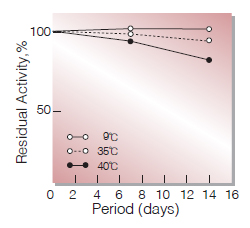
Fig.3. Stability (Liquid form)
(in 50 mM K-phosphate buffer,pH7.5)
-
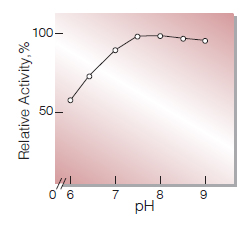
Fig.4. pH-Activity
37℃ ,10min-reaction in 50mM K-phoshate buffer
-
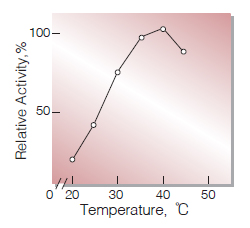
Fig.5.Temperature activity
10min-reaction in 50mM K-phosphate buffer, pH7.5
-
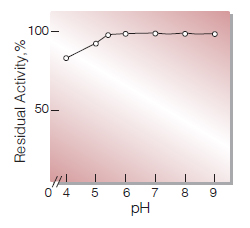
Fig.6.pH-Stability
25℃ 16hr-treatment with 50mM buffer solution: pH4.0-5.5,Acetate pH6.0-9.0, K-phosphate
-
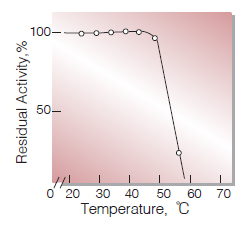
Fig.7. Thermal stability
30min-treament with 50mM K-phosphate buffer, pH7.5
活性測定法(Japanese)
1. 原理

生成した尿素のp-ジメチルアミノベンズアルデヒド(DAB)との縮合(Ehrich反応)生成物(黄色色素)を比色定量する。
2.定義
下記条件下で1分間に1マイクロモルの黄色色素を生成する酵素量を1単位(U)とする。
3.試薬
- 0.1Mクレアチン溶液〔1.49gのクレアチン(ナカライテスク製)を50mMリン酸緩衝液 pH7.5に溶解し,100 mLとする〕(用時調製)
- DAB溶液(2.0gのp-ジメチルアミノベンズアルデヒドを100 mLのジメチルスルホキシドに溶解させた後,濃塩酸15 mLを加える)
酵素溶液:酵素標品を予め氷冷した50mMリン酸緩衝液,pH7.5で溶解し,分析直前に同緩衝液で2.0~3.0U/mLに希釈する。
4.手順
1.試験管に基質溶液(A)1.0 mLを採り,37℃で約5分間予備加温する。
2.酵素溶液0.1 mLを加え,反応を開始する。
3.37℃で正確に10分間反応させた後,DAB溶液(B)2.0 mLを加えて反応を停止させる。
4.25℃で20分間放置後,435nmにおける吸光度を測定する (ODtest)。
5.盲検は基質溶液(A)1.0 mLを37℃で10分間放置後,DAB溶液(B)2.0mlを加えて混和し,次いで酵素溶液0.1mlを加えて調製する。以下同様に25℃で20分間放置後吸光度を測定する(ODblank)。
5.計算式
U/mL =
-
ΔOD(OD test−OD blank)×3.1(mL)×希釈倍率
0.321×1.0×10(分)×0.1(mL)
| = ΔOD×9.65×希釈倍率 | |
| U/mg | =U/mL×1/C |
| 0.321 | : 黄色色素のミリモル分子吸光係数(cm2/micromole) |
| 1.0 | : 光路長(cm) |
| C | : 溶解時の酵素濃度(c mg/mL) |
CONTACT
-
For inquiries and cosultations regarding our products, please contact us through this number.
- HEAD OFFICE+81-6-6348-3843
- Inquiry / Opinion
