- HOME
- Enzyme List
- GLA-111 GLUCOAMYLASE
-
GLA-111
GLUCOAMYLASE from Rhizopus sp.
PREPARATION and SPECIFICATION
| Appearance | White amorphous powder (salt-free), lyophilized |
|---|---|
| Activity | GradeⅠ 30 U/mg-solid or more |
PROPERTIES
| Stability | Stable at −20 ℃ for at least one year (Fig.1) |
|---|---|
| Molecular weight | approx. 70,0001) |
| Michaelis constants1) | 11 ± 1.1×10-4 M (Maltose), 3.6 ± 0.51×10-4 M (Maltotriose), 2.5 ± 0.33×10-4 M (Maltotetraose), 1.6 ± 0.02×10-4 M (Maltopentaose) |
| Structure | Glycoprotein[E 280 nm 1 cm (1 %)=14.5] 1 cm |
| Optimum pH | 4.5−5.0 (Fig.3) |
| Optimum temperature | 60 ℃ (Fig.4) |
| pH Stability | pH 4.0−8.5 (25 ℃, 20 hr) (Fig.5) |
| Thermal stability | below 45 ℃ (pH 5.5, 10 min) (Fig.6) |
| Substrate specificity1,2) | This enzyme completely hydrolyzes soluble starch, amylopectin, glycogen,α-orβ-limit dextrin, amylose, maltooligosaccharides and panose. |
APPLICATIONS
This enzyme is useful for structural analysis of carbohydrates and for enzymatic determination of α-amylase in combination with the related enzymes in clinical analysis.
ASSAY
Principle

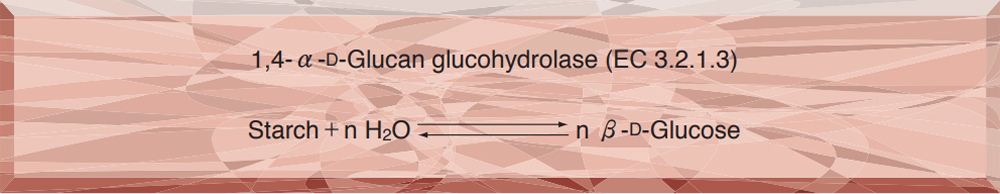
The formation of glucose is measured with reducing sugar as the index, by the modified Fehling-Lehmann-Schoorl method.
Unit definition
One unit causes the formation of ten milligrams of glucose in 30 minutes under the conditions detailed below.
Method
Reagents
| A. Starch solution | 1.0 %: Suspend 1.0 g of soluble starch (Merck) in 90 mL of H2O, dissolve by boiling for 3 min, and cool to room temperature. Add 5.0 mL of 1.0 M acetate buffer, pH 4.5, and make up to 100 mL with H2O (should be freshly prepared). |
|---|---|
| B. Alkaline solution | 100 g of NaOH, 365 g of potassium sodium tartrate tetrahydrate, per 1,000 mL of H2O |
| C. CuSO4 Solution | 7.0 % :70 g CuSO4・5H2O/1,000 mL of H2O |
| D. KI solution | 30 %: 300 g of KI per 1,000 mL of H2O (store in a brownish bottle) |
| E. H2SO4 Solution | 25 % |
| F. Na2S2O3 Solution | 50 mM: 49.638 g pf Na2S2O3・5H2O, 4.0 g of Na2CO3 (stabilizer) in 4,000 mL of H2O (store in a brownish bottle and keep for 3~4 days before use). |
| G. Enzyme diluent | 10 mM acetate buffer, pH 4.5 |
Procedure
1. Pipette 4.0 mL of substrate solution (A) into a test tube (32 φ× 200 mm) and equilibrate at 40 ℃ for approximately 5minutes.
| Concentration in assay mixture | |
|---|---|
| Acetate buffer | 42 mM |
| Starch | 0.8 % |
2. Add 1.0 mL of the enzyme solution* and mix.
3. After exactly 15 minutes at 40 ℃, add 2.0 mL of alkaline solution (B) to stop the reaction.
At the same time, prepare the blank by first mixing the substrate solution with 2.0 mL of alkaline solution after incubation for 15 min at 40 ℃, followed by addition of the enzyme solution.
4. Add 2.0 mL of CuSO4 solution (C) and, after covering the test tube with a marble (40 mmφ) to prevent evaporation, place the test tube in a boiling water bath.
5. After 20 minutes, remove the test tube from the boiling water bath and cool to room temperature under running water.
6. Add 2.0 mL each of KI solution (D) and H2SO4 solution (E) in this order.
7. Shake the test tube and determine the amount of residual Cu2+ by titration with Na2S2O3 solution (F).
8. Record the titers (mL) of the test (Δt) and the blank (Δb), and calculate the titration difference in mL (Δ sample: Δb−Δt).
*Dissolve the enzyme preparation in ice-cold distilled water and dilute to 0.4−1.5 U/mL with enzyme diluent (G), immediately before assay.
Calculation
Activity can be calculated by using the following formula :
Volume activity (U/mL) =
-
Δsample×30 min×df
Δglucose×15 min
=
-
Δsample
Δglucose
×2.0×df
Weight activity (U/mg) = (U/mL)×1/C
| Δglucose | : Titration difference (mL) for ten miligrams of glucose (Determine the titration difference by using glucose standard solution (5.0mg/mL) instead of the enzyme solution under the above assay conditions.) |
| df | : Dilution factor |
| C | : Enzyme concentration in dissolution (c mg/mL) |
REFERENCES
1) K.Hiromi, Y. Nitta, C.Numata and S.Ono; Biochim.Biophys.Acta, 302, 362 (1973).
2) J.Fukumoto; Protein, Nucleic Acid and Enzyme, 4, 3 (1959).
-
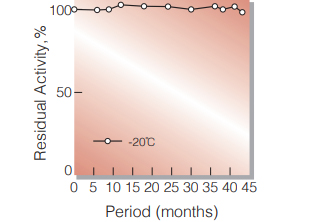
Fig.1. Stability (Powder form)
(kept under dry conditions)
-
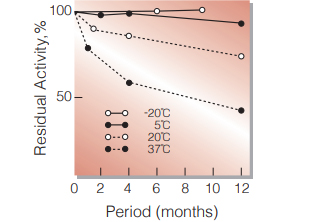
Fig.2. Stability (Powder form)
(kept under dry conditions)
-
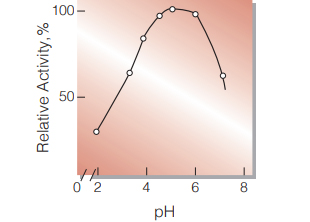
Fig.3. pH-Activity
40℃,15min-reaction in 50mM buffer solution: pH2.0,sodium acetate-HCI; pH3.0-6.0,acetate;pH6.0-7.0, phosphate
-
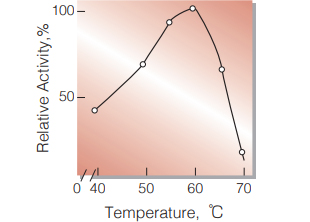
Fig.4.Temperature activity
15min-reaction in 50mM acetate buffer,pH4.5
-
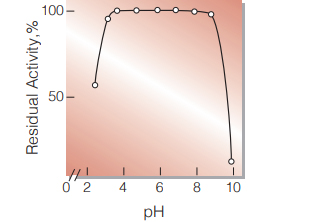
Fig.5.pH-Stability
25℃,20hr-treatment with 50mM buffer solution: pH3.0-6.0 acetate; pH6.0-9.0,phosphate;pH9.0-10.0, borate
-
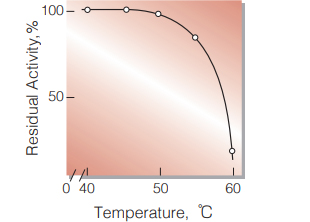
Fig.6.Thermal stability
10min-treatment with 50mM acetate buffer,pH5.5
活性測定法(Japanese)
1. 原理

グルコース(還元糖)の生成量をフェーリング・レーマン・ショール変法で測定する。
2.定義
下記反応条件下で30分間に10mgのグルコースを生成する酵素量を1単位(U)とする。
3.試薬
- 1.0 %可溶性澱粉溶液〔1.0gの可溶性澱粉 (Merck製)を90 mLの蒸留水に懸濁後,約3分間煮沸溶解する。室温迄冷却後,1.0M酢酸緩衝液, pH4.5を5.0 mL添加し,最終液量を蒸留水で100 mLとする〕(用時調製)
- ロッシェル塩アルカリ溶液(100gのNaOH及び 365gの酒石酸カリウム・ナトリウム塩・4H2Oを蒸留水に溶解し,1000 mLとする)
- 7.0 %硫酸銅溶液(70gのCuSO4・5H2Oを蒸留水に溶解し,1,000 mLとする)
- 30 %ヨードカリ溶液(300gのKIを蒸留水に溶解し, 1,000 mLとする)(褐色瓶中で保存)
- 25 %硫酸溶液
- 50mMチオ硫酸ナトリウム溶液〔49.638gの Na2S2O3・5H2O及び4.0gのNa2CO3(安定化剤) を蒸留水に溶解し,4,000 mLとする〕(褐色瓶中で保存し,調製後3~4日放置して使用する)
酵素溶液:酵素標品を予め氷冷した蒸留水で溶解し, 分析直前に10mM酢酸緩衝液 pH4.5で0.4 ~1.5 U/mLに希釈する。
4.手順
1.試験管(32φ×200mm)に基質溶液 (A)4.0 mLを採り, 40℃で約5分間予備加温する。
2.酵素溶液を1.0 mLを加え,反応を開始する。
3.40℃で正確に15分間反応させた後,ロッシェル塩アルカリ溶液 (B) 2.0 mL加えて反応を停止させる。
4.硫酸銅溶液 (C)を2.0 mL加え,試験管上に40mmφのガラス玉をのせ(蒸発防止)沸騰浴中で20分間煮沸する。
5.流水中で室温迄冷却する。
6.ヨードカリ溶液 (D) 2.0 mL及び硫酸溶液 (E) 2.0 mLをこの順序に加える。
7.よく混和した後,チオ硫酸ナトリウム溶液 (F)で滴定する。→(反応滴定値)
8.盲検は基質溶液 (A) 4.0 mLを40℃で15分間放置後, ロッシェル塩アルカリ溶液(B) 2.0 mLを加えて混和し,次いで酵素溶液1.0 mLを加えて調製する。以下手順④ ~⑦を操作して滴定値を求める。→(盲検滴定値)
5.計算式
U/mL =
-
(盲検滴定値−反応滴定値)×30(分)×希釈倍率
標準滴定値×15(分)
-
=
-
(盲検滴定値−反応滴定値)
標準滴定値
-
×2.0×希釈倍率
U/mg =U/mL×1/C
| 標準滴定値 | : 酵素溶液の代りにグルコース標準溶液 (5.0㎎/㎖)を用いて上記手順に従って操作し,グルコース10mgに相当する滴定値を算出する(内部標準) |
| C | : 溶解時の酵素濃度(c mg/mL) |
CONTACT
-
For inquiries and cosultations regarding our products, please contact us through this number.
- HEAD OFFICE+81-6-6348-3843
- Inquiry / Opinion
