- HOME
- Enzyme List
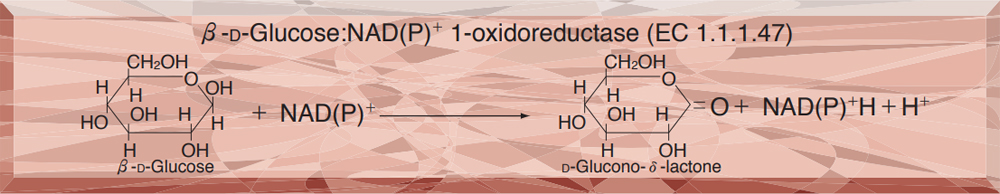
- GLD-311 GLUCOSE DEHYDROGENASE (NAD(P)-dependent)
GLD-311
GLUCOSE DEHYDROGENASE (NAD(P)-dependent) from Microorganism
PREPARATION and SPECIFICATION
| Appearance | White amorphous powder, lyophilized. | |
|---|---|---|
| Activity | GradeⅢ 250 U/mg-solid or more | |
| Contaminants | NADH oxidase | ≤1.0×10-3 % |
| α-Glucosidase | ≤1.0×10-3 % | |
| Glucose-6-phosphate dehydrogenase | ≤1.0×10-3 % | |
PROPERTIES
| Stability | Stable at −20 ℃ for at least one year (Fig.1) | |
|---|---|---|
| Molecular weight | approx. 101,000 (Gel filtration) | |
| Isoelectric point | 4.5 | |
| Michaelis constants | NAD+linked | 1.38×10-2 M (D-Glucose) 3.09×10-4 M (NAD+) |
| NADP+linked | 1.25×10-2 M (D-Glucose) 4.07×10-5 M (NADP+) | |
| Inhibitors | Ag+, Hg2+, Monoiodoacetate | |
| Optimum pH | 9.0 (Fig.4) | |
| Optimum temperature | 55 ℃ (Fig.5) | |
| pH Stability | pH 6.0−7.5 (20 ℃, 16 hr) (Fig.6) | |
| Thermal stability | 45 ℃ (15 min-treatment with 50 mM K-phosphate buffer, pH 7.0)(Fig.7) | |
| Substrate specificity | Specific for β-D-Glucose or 2-Deoxy-glucose (Table.1) (Either NAD+ or NADP+ serves as coenzyme.) | |
APPLICATIONS
This enzyme is useful for enzymatic determination of D-glucose.
ASSAY
Principle

The formation of NADH is measured at 340 nm by spectrophotometry.
Unit definition
One unit causes the formation of one micromole of NADH per minute under the conditions detailed below.
Method
Reagents
| A. Tris-HCl buffer, pH 8.0 | 0.1 M |
|---|---|
| B. D-Glucose solution | 1.5 M |
| C. β-NAD+ solution | 80 mg/mL |
| D. Enzyme diluent | 50 mM K-phosphate buffer, pH 7.0 contg. 0.1 % BSA |
Procedure
1.Prepare the following reaction mixture in a cuvette (d = 1.0cm) and equilibrate at 37 ℃ for approximately 5 minutes.
| 2.6 mL | Tris-HCl buffer, pH 8.0 | (A) |
| 0.3 mL | Substrate solution | (B) |
| 0.1 mL | β-NAD+ solution | (C) |
| Concentration in assay mixture | |
|---|---|
| Tris-HCl buffer | 85.25 mM |
| D-Glucose | 147.54 mM |
| NAD+ | 3.66 mM |
2.Add 0.05 mL of the enzyme solution* and mix by gentle inversion.
3.Record the increase in optical density at 340 nm against water for 2 to 5 minutes with a spectrophotometer thermostated at 37 ℃, and calculate the ΔOD per minute from the initial linear portion of the curve (ΔOD test).
At the same time, measure the blank rate (ΔOD blank) using the same method as the test except that the enzyme diluent (D) is added instead of the enzyme solution.
*Dissolve the enzyme preparation in ice-cold enzyme diluent (D), dilute to 0.8−1.2 U/mL with the same buffer and store on ice.
Calculation
Activity can be calculated by using the following formula :
Volume activity (U/mL) =
-
ΔOD/min (ΔOD test−ΔOD blank)×Vt×df
6.22×1.0×Vs
= ΔOD/min×9.807×df
Weight activity (U/mg) = (U/mL)×1/C
| Vt | : Total volume (3.05 mL) |
| Vs | : Sample volume (0.05 mL) |
| 6.22 | : Millimolar extinction coefficient of NADH under the assay conditions (cm2/micromole) |
| 1.0 | : Light path length (cm) |
| df | : Dilution factor |
| C | : Enzyme concentration in dissolution (c mg/mL) |
Table 1. Substrate Specificity of Glucose dehydrogenase
-
Substrate (150mM) Relative activity(%) D-Glucose 100.0 L-Glucose 0.0 D-Xylose 16.2 2-Deoxy-glucose 127.0 L-Sorbose 0.0 D-Mannose 5.1 D-Fructose 0.0 -
Substrate (150mM) Relative activity(%) Galactose 1.7 D-Lactose 1.5 D-Sorbitole 0.0 D-Mannitol 0.0 Sucrose 0.0 Inositol 0.0 Maltose 1.4
Table 2. Effect of Various Chemicals on Glucose dehydrogenase
[The enzyme dissolved in 50 mM K-phosphate buffer, pH 7.0 (2.8 U/mL) was incubated with each chemical for 1 hr at 30 ℃.]
-
Chemical Concn.(mM) Residual
activity(%)None - 100 Metal salt 2.0 AgNO3 7.1 Ba(OAc)2 98.2 CaCl2 98.9 Cd(OAc)2 96.6 CoCl2 96.4 CuSO4 99.5 FeCl3 98.1 FeSO4 96.6 HgCl2 5.9 MgCl2 101.5 MnCl2 100.9 NiCl2 93.4 Pb(OAc)2 99.8 ZnSO4 102.1 -
Chemical Concn.(mM) Residual
activity(%)KF 2.0 98.7 NaF 10.0 100.6 NaN3 20.0 101.6 NEM 2.0 97.6 MIA 2.0 0.4 IAA 2.0 92.2 EDTA 5.0 107.2 (NH4)2SO4 20.0 96.0 Borate 20.0 101.4 o-Phenanthroline 2.0 97.7 α,α′-Dipyridyl 1.0 100.3 Urea 2.0 122.5 Guanidine 2.0 99.2 Hydroxylamine 2.0 107.2
Ac, CH3CO; NEM, N-Ethylmaleimide; MIA, Monoiodoacetate; IAA, lodoacetamide; EDTA, Ethylenediaminetetraacetate.
-
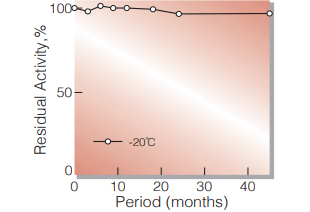
Fig.1. Stability (Powder form)
(kept under dry conditions)
-
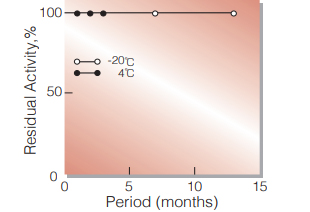
Fig.2. Stability (Powder form)
(kept under dry conditions)
-
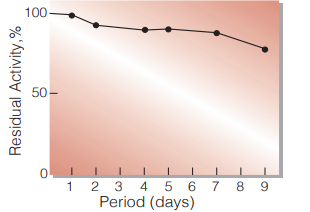
Fig.3. Stability (Liquid form)
25 ℃,in 83 mM Tris-HCI buffer solution pH 8.0(contg.3.7 mM β-NAD,40 U/mL mutarotase) enzyme concn.:300 U/mL
-
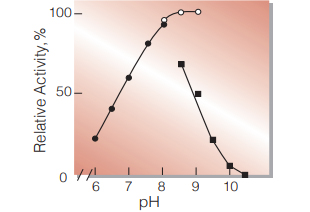
Fig.4. pH-Activity
37 ℃,5 min-reaction in 80 mM buffer solution
●:pH 6.0-8.0 K-phosphate
○:pH 8.0-9.0,Tris-HCI
■:pH 8.5-10.5 Carbonate -
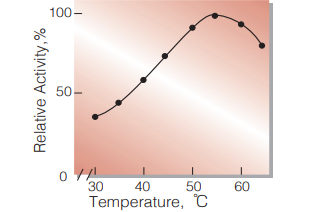
Fig.5. Temperature activity
(in 80 mM Tris-HCI buffer, pH 8.0)
-
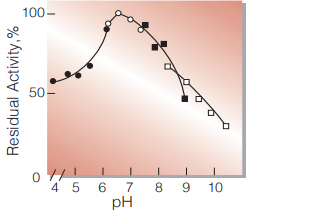
Fig.6. pH-Stability
20 ℃,16 hr with 0.1 M buffer solution
●:pH 4.0-6.0 acetate
○:pH 6.0-8.0 K-phosphate
■:pH 7.5-9.0 Tris-HCI
□:pH 8.5-10.5 carbonate
enzyme concn.:10 U/mL -
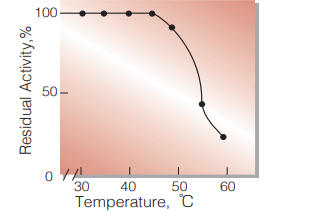
Fig.7. Thermal stability
15 min-treatment with 50 mM K-phosphate buffer pH 7.0 enzyme concn.: 12 U/mL
活性測定法(Japanese)
1. 原理

NADHの生成量を340nmの吸光度の変化で測定する。
2.定義
下記条件下で1分間に1マイクロモルのNADHを生成する酵素量を1単位(U)とする。
3.試薬
- 0.1M Tris-HCl緩衝液,pH8.0
- 1.5M D-Glucose水溶液
- 80mg/mLNAD+ 水溶液 (用時調製)
酵素溶液:酵素標品を予め氷冷した0.1 %BSAを含む 50mMK-リン酸緩衝液,pH 7.0で溶解し,分析直前に同緩衝液で0.8〜1.2U/ mLに希釈する。
4.手順
1.下記反応混液をキュベット(d=1.0cm)に調製し,37℃で約5分間予備加温する。
| 2.6 mL | Tris-HCl緩衝液 | (A) |
| 0.3 mL | D-グルコース水溶液 | (B) |
| 0.1 mL | NAD+ 水溶液 | (C) |
2.酵素溶液0.05 mLを添加し,ゆるやかに混和後,水を対 照に37℃に制御された分光光度計で340nmの吸光度変化を5分間記録し,その2〜5分の直線部分から1分間当りの吸光度変化を求める(ΔODtest)。
3.盲検は酵素溶液の代わりに酵素希釈液(リン酸緩衝液,pH 7.0)を0.05 mL加え,上記同様に操作を行って1分間当りの吸光度変化を求める。(ΔODblank)
5.計算式
-
U/mL =
-
ΔOD/min (ΔOD test−ΔOD blank)×3.05×希釈倍率
6.22×1.0×0.05
| = ΔOD/min×9.807×希釈倍率 | |
| U/mg | =U/mL×1/C |
| 6.22 | : NADHのミリモル分子吸光係数 (cm2/micromole) |
| 1.0 | : 光路長(cm) |
| C | : 溶解時の酵素濃度(c mg/mL) |
CONTACT
-
For inquiries and cosultations regarding our products, please contact us through this number.
- HEAD OFFICE+81-6-6348-3843
- Inquiry / Opinion
