- HOME
- Enzyme List
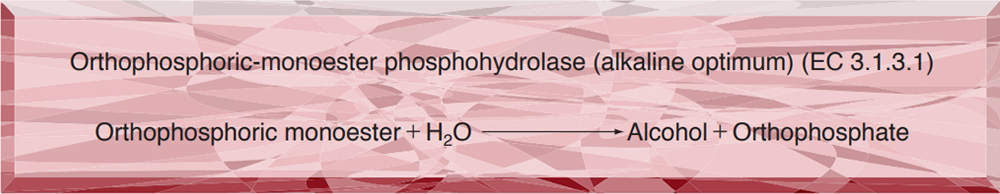
- LPP-229 ALKALINE PHOSPHATASE
LPP-229
ALKALINE PHOSPHATASE from Microorganism
PREPARATION and SPECIFICATION
| Appearance | Transparent liquid | |
|---|---|---|
| Activity | GradeⅡ 30,000 U/mL or more | |
| Contaminants | Adenosine deaminase | ≤ 1.0×10-4 % |
| Phosphodiesterase | ≤ 3.0×10-3 % | |
PROPERTIES
| Stability | Stable at 4 ℃ |
|---|---|
| Molecular weight | approx. 104,000 |
| Optimum pH | 9.5(Fig. 1) |
| Optimum temperature | ≧ 60 ℃ (Fig. 2) |
| pH Stability | pH 5.5-10.4 (25 ℃, 16 hr)(Fig. 3) |
| Thermal stability | below 65 ℃ (pH 7.0, 60 min) (Fig. 4) |
APPLICATIONS
This enzyme is useful in molecular biology.
ASSAY
Principle
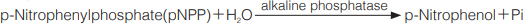
The formation of p-nitrophenol is measured at 405 nm by spectrophotometry.
Unit definition
One unit causes the formation of one micromole of p-nitrophenol per minute under the conditions detailed below.
Method
Reagents
| A. Diethanolamine buffer | 1 M: Dilute 9.66 mL of diethanolamine (MW = 105.14) in 60 mL of H2O, add 5 mL of 0.1 M MgCl2, and, after adjusting the pH to 9.8 with 2 N HCl, make up to 100 mL with H2O (should be freshly prepared). |
|---|---|
| B. pNPP solution | 0.674 M: 2.5g of p-nitrophenylphosphate disodium salt (MW = 371.16) in 10 mL of diethanolamine buffer (A). Should be freshly prepared. |
| C. Enzyme diluent | 30 mM Triethanolamine, 1 mM MgCl2, 0.1 mM ZnCl2, 0.5 % sodium cholate, pH 7.6 |
Procedure
1.Prepare the following working solution (30.5 mL) in a brownish bottle and store on ice (should be freshly prepared).
| 30 mL | Diethanolamine buffer | (A) |
| 0.5 mL | pNPP solution | (B) |
| Concentration in assay mixture | |
|---|---|
| Diethanolamine buffer | 0.97 M |
| p-Nitrophenylphosphate | 11 mM |
| MgCl2 | 4.8 mM |
2.Pipette 3.0 mL of working solution into a cuvette (d = 1.0 cm) and equilibrate at 37 ℃ for approximately 5 minutes.
3.Add 0.1 mL of the enzyme solution* and mix by gentle inversion.
4.Record the increase in optical density at 405 nm against water for 3 to 5 minutes with a spectrophotometer thermostated at 37 ℃, and calculate the ∆OD per minute from the initial linear portion of the curve (∆OD test).
At the same time, measure the blank rate (∆OD blank) using the same method as the test except that the enzyme diluent (C) is added instead of the enzyme solution.
*Dilute the enzyme preparation to 0.1-0.3 U/mL with ice-cold enzyme diluent (C), immediately before the assay.
Calculation
Activity can be calculated by using the following formula:
Volume activity (U/mL) =
-
∆OD/min(∆OD test-∆OD blank)×Vt×df
18.5×1.0×Vs
= ∆OD/min×1.676×df
Weight activity (U/mg) = (U/mL)×1/C
| Vt | : Total volume (3.1 mL) |
| Vs | : Sample volume (0.1 mL) |
| 18.5 | : Millimolar extinction coefficient of p-Nitrophenol under the assay condition (cm2/micromole) |
| 1.0 | : Light path length (cm) |
| df | : Dilution factor |
-
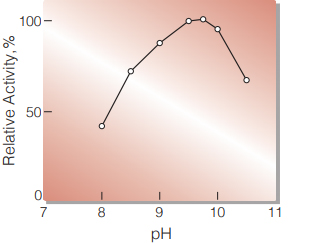
Fig.1 pH-Activity
(in 1M Diethanolamine buffer, pH 8-10.5)
-
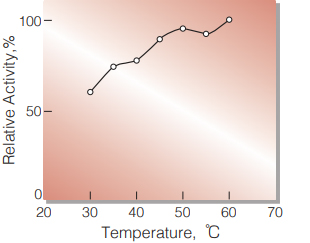
Fig.2 Temperature activity
(in 1M Diethanolamine buffer, pH 10.25 )
-
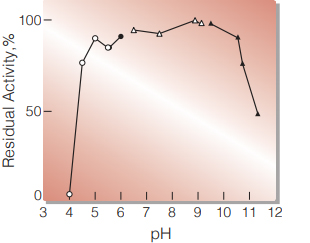
Fig.3 pH-Stability
25 ℃, 16 hr-treatment with 0.1 M buffer solution: pH 4-6, dimethylglutaric acid-NaOH; pH 6-8, K-phosphate; pH 8-9, Tris-HCl; pH 9-10, glycine-NaOH. Enzyme concentration: 10 U/mL
-
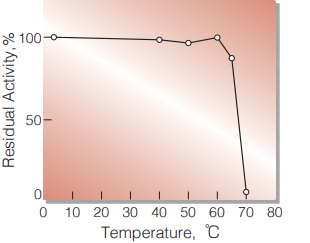
Fig.4 Thermal stability
15 min-treatment with 50 mM K-phosphate buffer, pH 7.0. Enzyme concentration: 10 U/mL
活性測定法(Japanese)
1. 原理
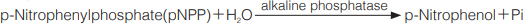
p-Nitrophenolの生成量を405nmにおける吸光度の変化で測定する。
2.定義
下記条件下で1分間に1マイクロモルのp-Nitrophenolを生成する酵素量を1単位(U)とする。
3.試薬
- 1Mジエタノールアミン緩衝液、pH9.8 [9.66 mLのジエタノールアミン (MW=105.14)を蒸留水60 mLで希釈後、0.1M MgCl2 5 mLを添加する。さらに2.0N HClで37℃におけるpHを9.8に調整し、最終液量を100 mLとする] (用時調製)
- 0.674M pNPP溶液 [2.5gのp-ニトロフェニルリン酸二ナトリウム塩 (MW=371.16)を10 mLの緩衝液Aに溶解する] (用時調製)
- 酵素溶液: 30mM トリエタノールアミン、1mM MgCl2、0.1mM ZnCl2、0.5 % Sodium cholate, pH7.6で反応直前に0.1~0.3U/mLに希釈する。
4.手順
1.下記反応混液(30.5mL)を調製する。
| 30 mL | ジエタノールアミン緩衝液 | (A) |
| 0.5 mL | pNPP溶液 | (B) |
2.3.0 mLの反応混液をキュベット(d=1.0cm)に移し、37℃で約5分間予備加温する。
3.酵素溶液0.1 mLを添加し、ゆるやかに混和する。
4.水を対照に37℃に制御された分光光度計で405nmの吸光度変化を3~5分間記録し、その初期直線部分から1分間当たりの吸光度変化を求める(∆OD test)。盲検は酵素溶液に代えて酵素希釈液を加え、上記同様に操作を行って1分間当たりの吸光度変化を求める(∆OD blank)。
5.計算式
U/mL =
-
ΔOD/min (ΔOD test - ΔOD blank) × 3.00(mL) × 希釈倍率
18.5 × 1.0 × 0.1 (mL)
| = ∆OD/min × 1.676 × 希釈倍率 | |
| Vt | : 総液量 (3.1 mL) |
| Vs | : 試料総量 (0.1 mL) |
| 18.5 | : p-Nitrophenolのミリモル分子吸光係数 |
| 1.0 | : 光路長(cm) |
CONTACT
-
For inquiries and cosultations regarding our products, please contact us through this number.
- HEAD OFFICE+81-6-6348-3843
- Inquiry / Opinion
