- HOME
- Enzyme List
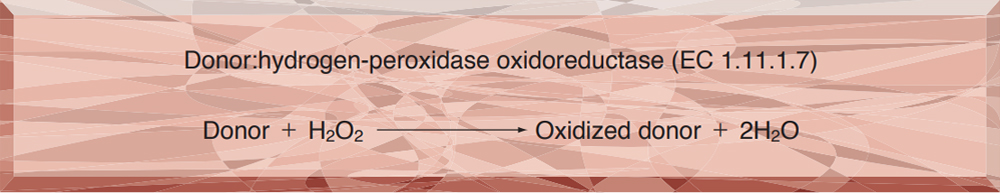
- PEO-701 PEROXIDASE
PEO-701
PEROXIDASE from Horseradish
PREPARATION and SPECIFICATION
| Appearance | Reddish-brown amorphous powder, lyophilized | |
|---|---|---|
| Activity | GradeⅦ 60 Purpurogallin U/mg-solid or more (RZ ≥ 0.6) |
|
PROPERTIES
| Stability | Stable at −20 ℃ |
|---|---|
| Molecular weight | approx. 40,000 (by gel filtration) |
| Structure | Glycoprotein with one mole of protohaemin Ⅸ |
| Inhibitors | Cyanide, sulfide, fluoride, azide |
| Optimum pH | 8.0 (Fig.2) |
| pH Stability | 5.0−10.0 (Fig.3) |
| Thermal stability | below 60 ℃ (Fig.4) |
APPLICATIONS
This enzyme is useful for enzymatic determination of H2O2 in clinical analysis.
ASSAY
Principle

The formation of purpurogallin is measured at 420 nm by spectrophotometry.
Unit definition
One unit causes the formation of one milligram of purpurogallin in 20 seconds under the conditions detailed below.
Method
Reagents
| A. Pyrogallol solution | 5 % (W/V)(Should be prepared fresh). |
|---|---|
| B. H2O2 solution | 0.147 M[Dilute 1.67 mL of 30 % (W/V) H2O2 to 100 mL with H2O](Should be prepared fresh) |
| C. Phosphate buffer, pH6.0 | 0.1 M |
| D. H2SO4 solution | 2.0 N |
Procedure
1.Prepare the following reaction mixture in a test tube (32 φ × 200 mm) and equilibrate at 20 ℃ for about 5 minutes.
| 14.0 mL | H2O | |
|---|---|---|
| 2.0 mL | Pyrogallol solution | (A) |
| 1.0 mL | H2O2 solution | (B) |
| 2.0 mL | Phosphate buffer, pH 6.0 | (C) |
| Concentration in assay mixture | |
|---|---|
| Phosphate buffer | 15 mM |
| Pyrogallol | 40 mM |
| H2O2 | 7.4 mM |
2.Add 1.0 mL of the enzyme solution* and mix.
3.After exactly 20 seconds at 20 ℃, add 1.0 mL of 2.0 N H2SO4 solution (D) to stop the reaction.
4.Extract the produced purogallin from the above stopped reaction mixture in five times with 15 mL portions of ether and fill up the combined ether extracts to 100 mL with fresh ether.
5.Measure the optical density at 420 nm against water (OD test).
At the same time, prepare the blank by first mixing the reaction with 1.0 mL of 2.0 N H2SO4 solution (D) after 20 sec-incubation at 20 ℃, followed by the addition of the enzyme solution and extracting with ether by the same procedure as the test (OD blank).
*Dissolve the enzyme preparation in ice-cold 0.1 M phosphate buffer, pH 6.0 (C), dilute to 3.0−6.0 purpurogallin U/mL with the same buffer and store on ice.
Calculation
Activity** can be calculated by using the following formula :
Volume activity (U/mL) =
-
ΔOD (OD test−OD blank)×Vt×df
0.117×Vs
= ΔOD×8.547×df
Weight activity (U/mg) = (U/mL)×1/C
| Vs | : Sample volume (1.0 mL) |
| 0.117 | : Optical density at 420 nm corresponding to 1 mg% of Purpurogallin in ether |
| df | : Dilution factor |
| C | : Enzyme concentration in dissolution (c mg/mL) |
| **One purpurogallin unit is equivalent to 13.5 international units determined with o-dianisidine at 25 ℃. | |
-
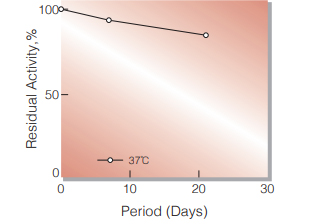
Fig.1. Stability (Powder form)
(kept under dry conditions)
-
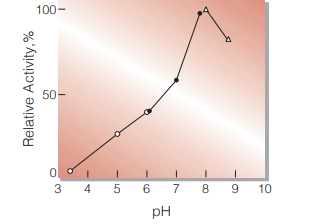
Fig.2. pH-Activity
37 ℃, in 0.1 M buffer solution; pH 3.3-6, Acetate; pH 6-7.5, K-Phosphate; pH 7.5-9, Tris-HCl
-
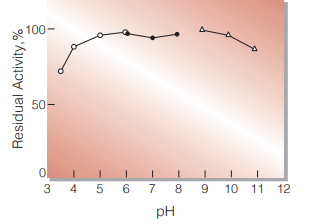
Fig.3. pH-Stability
25 ℃, 20 hr-treatment with 0.1 M buffer solution; pH 3-6, Acetate; pH 6-8, K-Phosphate; pH 9-11, Glycine-NaOH
-
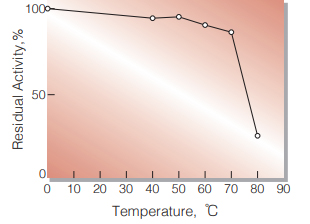
Fig.4. Thermal stability
10 min-treatment with 0.05 M K-Phosphate buffer, pH 6.0 Enzyme concentration : 2 U/mL
活性測定法(Japanese)
1. 原理

生成するPurpurogallinをエーテル抽出し,420nmの吸光度の変化で測定する。
2.定義
下記条件下で20秒間に1.0mgのPurpurogallinを生成する酵素量を1Purpurogallin単位(U)とする。
3.試薬
- 5 %(W/V)ピロガロール水溶液(用時調製)
- 0.147M H2O2水溶液〔30 %(W/V)H2O2溶液 1.67 mLを蒸留水で希釈して100 mLとする〕 (用時調製)
- 0.1Mリン酸緩衝液,pH6.0(反応混液及び酵素希釈用)
- 2.0N H2SO4溶液
酵素溶液:酵素標品を予め氷冷した0.1Mリン酸緩衝液, pH6.0で溶解し,同緩衝液で3.0〜 6.0Purpurogallin U/mLに希釈して氷冷保存する。
4.手順
1.試験管(32φ×200mm)に下記反応混液を調製し, 20℃で約5分間予備加温する。
| 14.0 mL | 蒸留水 | |
| 2.0 mL | ピロガロール水溶液 | (A) |
| 1.0 mL | H2O2水溶液 | (B) |
| 2.0 mL | リン酸緩衝液 | (C) |
2.酵素溶液1.0 mLを加え,反応を開始する。
3.20℃で正確に20秒間反応させた後, H2SO4溶液 (D)1.0 mLを加えて反応を停止させる。反応停止後の混液から生成したPurpurogallinをエーテル15 mLで抽出する。この操作を5回繰り返し,抽出液を合わせ,更にエーテルを加えて全量を100 mLにする。この液につき420nmにおける吸光度を測定する(OD test)。
4.盲検は反応混液①を20℃で20秒間放置後, H2SO4 溶液(D)を加えて混和し,次いで酵素溶液1.0 mLを加えて調製する。この液につき上記同様にエーテル抽出を行って吸光度を測定する(ODblank)。
5.計算式
U/mL =
-
ΔOD (OD test−OD blank)×希釈倍率
0.117×1(mL)
| = ΔOD×8.547×希釈倍率 | |
| U/mg | = U/mL×1/C |
| 0.117 | : 1 mg% Purpurogallinエーテル溶液の420nmにおける吸光度 |
| C | : 溶解時の酵素濃度(c mg/mL) |
| (注)1Purpurogallin単位は13.5国際単位(o-dianisidine を基質とし, 25℃の反応条件下)に相当する。 | |
CONTACT
-
For inquiries and cosultations regarding our products, please contact us through this number.
- HEAD OFFICE+81-6-6348-3843
- Inquiry / Opinion
