This content is for medical workers.
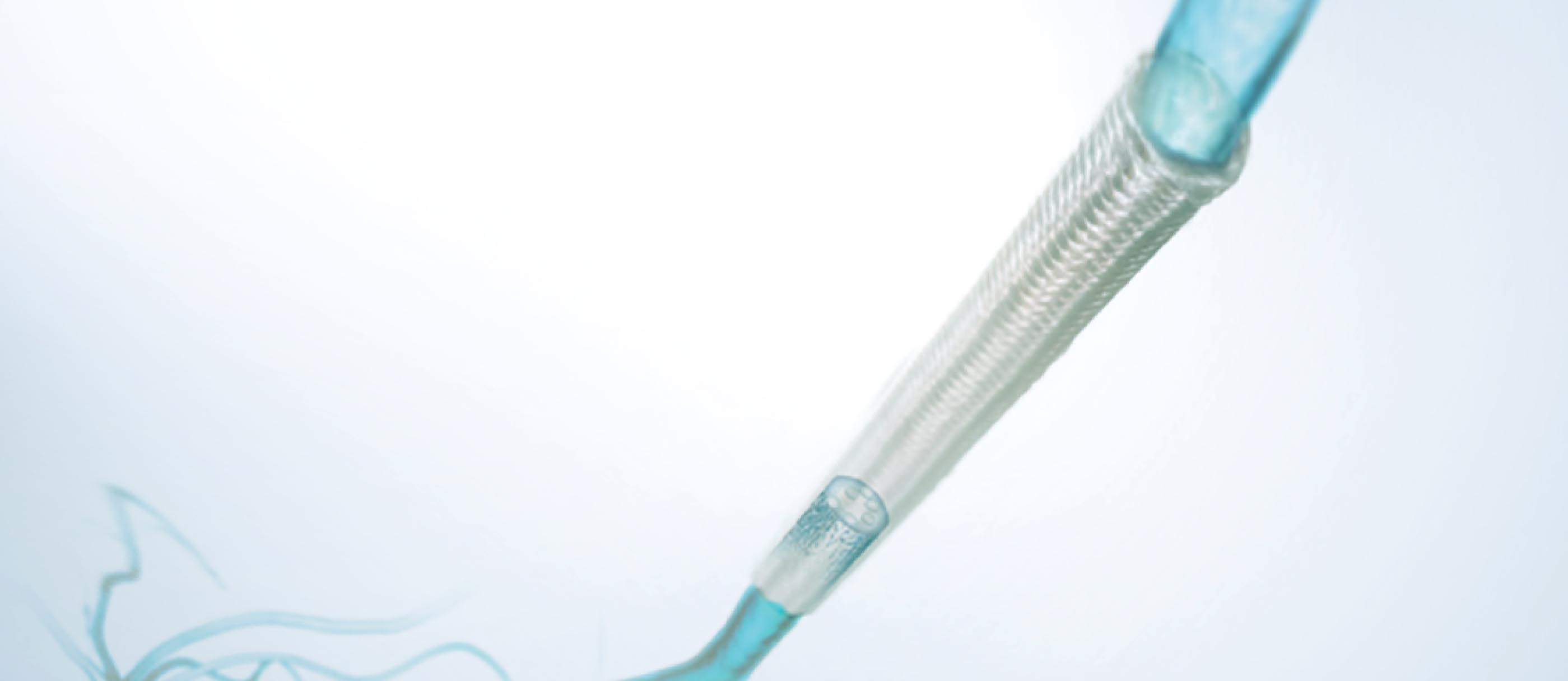
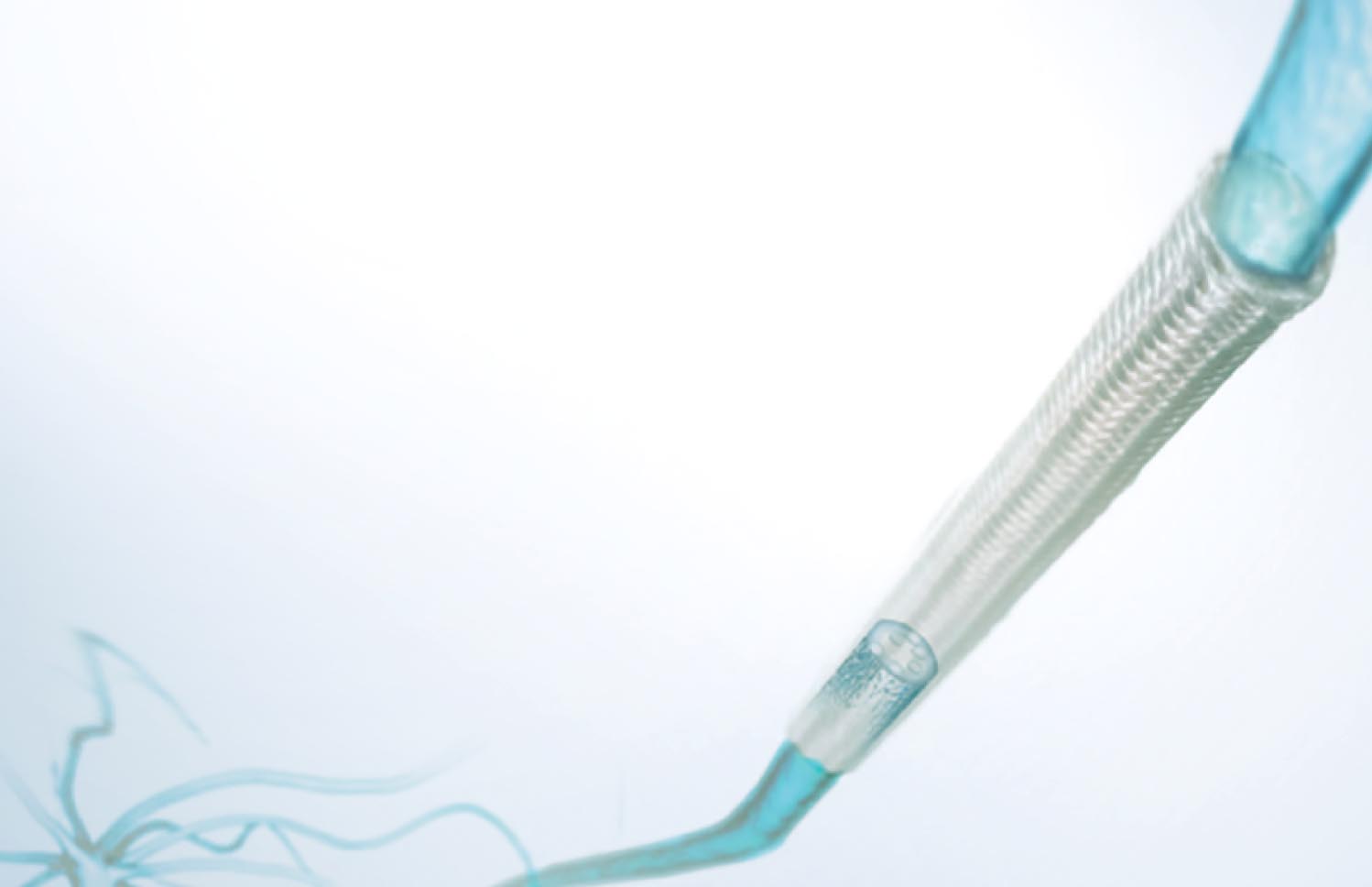
A new bridge for reconstructing damaged nerves
Nerve regeneration guidance conduit
510(k) No. K152967
Introduction of main indication sites / cases / nerves
Torso
Cavernosal nerve,
Intercostal nerve,
Other
Upper extremities
Radial nerve,
Median nerve,
Ulnar nerve,
Interosseous nerve,
Other
Head and neck
Facial nerve, Recurrent laryngeal nerve, Accessory nerve, Lingual nerve, Inferior alveolar nerve, Chorda tympani, Other
Lower extremities
Sural nerve, Peroneal nerve, Tibial nerve, Obturator nerve, Plantar nerve, Other
-

Promotes nerve
regenerationActively promotes the reconstruction of peripheral nerves. It is expected to provide the same level of treatment as "autologous nerve transplantation" and "nerve suture", expanding the options for nerve treatment.
-

Shorten the scope
and time of surgeryAs there is no need to harvest the nerve from a healthy site as in autologous nerve transplantation, it contributes to shortening the operation time and reducing the burden on the patient.
-

Installation of equipment
/ Smooth to useIt does not require special surgical equipment and can be used in primary emaergency hospitals.As the first nerve regeneration guidance conduit approved in Japan, we will continue to connect nerve treatment to tomorrow.
Manufacturer

Manufactured by
TOYOBO CO., LTD.
2-1-1, Katata, Otsu, Shiga 520-0292, Japan
https://www.toyobo-global.com/
Be sure to read the Directions for Use before using this product.
The specifications and appearance of this product are subject to change without notice for improvement.
The NerbridgeⓇ is a registered trademark of TOYOBO CO., LTD., Japan which indicates its nerve regeneration guidance conduit.
