This content is for medical workers.
How to use
Product Information
How to use
-
1. Open
Open the NerbridgeⓇ.
* The recommended size is 0.5mm to 1.0mm larger than the thickness of the autologous nerve.
-
Confirm the size of the device to be used and remove the Outer pouch from the outer box.
-
Open the Outer pouch and take the inner foil pouch.
(Please note that the outer pouch is for protection only and is not a sterile barrier. Only contents of the inner foil pouch are sterile.) -
Clean field
-
Carefully transfer the tray and NerbridgeⓇ into the clean field.
-
Remove the NerbridgeⓇ from the tray.
-
-
-
2. Preparation
Follow standard procedures for exposure and mobilization of the severed nerve. Determine the nerve diameter in millimeters (mm) using a suitable measuring instrument. Select a NerbridgeⓇ of sufficient diameter to allow easy insertion of the nerve stumps into NerbridgeⓇ.
Account for normal edema following traumatic nerve injury. Carefully transfer NerbridgeⓇ into the clean field. Hydrate NerbridgeⓇ in sterile saline for 3 minutes or more before use.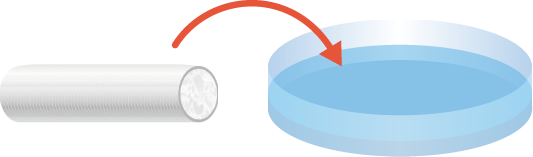
-
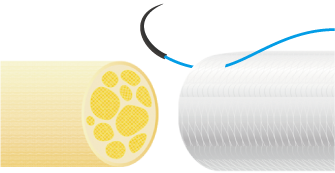
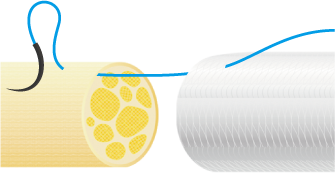
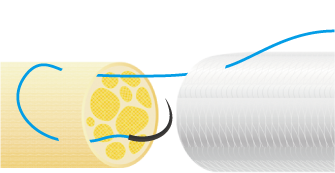
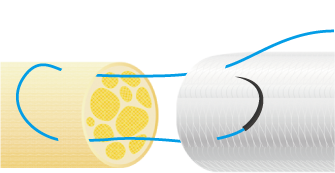
3. Suturing Procedure
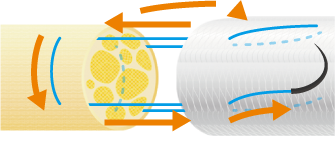
Using 6-0 to 10-0 non-absorbable monofilament nylon or polypropylene sutures, suture the proximal side first. Pass the suture through the wall of NerbridgeⓇ at a point about 1-2 mm from its end, transversing from the outside to the inside. Next, pass the suture through the epineurium of one nerve stump so that the tip of the needle exits 1-2 mm from the tip of the nerve stump. Reverse the suture and pass it through the epineurium of the nerve stump closer to the operator and away from where the needle exited. Take care not to damage any axons when suturing the epineurium. Next, pass the suture back through the wall of NerbridgeⓇ this time from the inside to the outside.
Then slowly rotate both the nerve stump and NerbridgeⓇ by 180 degrees, and suture the opposite side of NerbridgeⓇ and the nerve stump in the same manner as just described. When rotating NerbridgeⓇ, be careful not to cross the thread.
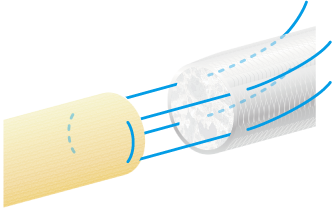
After this, pull the suture such that the nerve stump is gently drawn into the NerbridgeⓇ lumen.
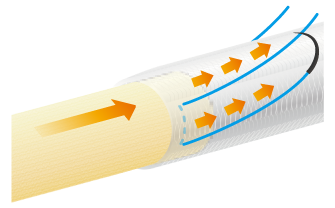
The approximate length of the nerve stump to be inserted into NerbridgeⓇ should be greater than or equal to the inner diameter of the NerbridgeⓇ lumen. A secure knot must be made in the suture, taking care not to apply tension on the suture itself.
NerbridgeⓇ must be long enough to allow each nerve stump to be drawn into the NerbridgeⓇ lumen at a distance greater than or equal to the inner diameter of NerbridgeⓇ. If needed, NerbridgeⓇ may be cut to an appropriate length.The suturing on the distal side should be done in the same manner as on the proximal side.

In addition, NerbridgeⓇ and the nerve should be ligated at multiple points and secured with sutures. If the tube falls out of the nerve stump, neuroma may develop.
-
Postoperative Procedure
Immobilize the repair area of the limb using standard procedures for immobilization following any peripheral nerve repair.
When NerbridgeⓇ is implanted close to a joint, immobilize the limb for at least 1 week. When starting rehabilitation pay attention to the positional relationship between the implanted site and the joint, in addition to the surrounding soft tissue.
Note: Proceed to rehabilitation depending on the condition of scars, the presence or absence of subjective and objective symptoms, the results of ultrasound imaging and a physical examination.
Storage
Store at temperature 1-30℃ / 33.8-86.0°F. Avoid excessive heat or humidity.
Product lineup
4 types of inner diameter size
| Catalog number | RN01025E | RN02025E | RN03025E | RN04025E |
|---|---|---|---|---|
| Inner diameter(mm) | 1.0 | 2.0 | 3.0 | 4.0 |
| Length(mm) | 25 | |||
* The recommended size for use is +0.5mm to +1.0mm above the thickness of the autologous nerve.
Directions for Use
Description
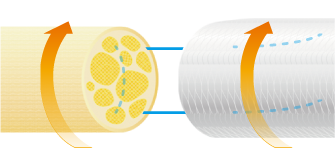
NerbridgeⓇ is a product composed of polyglycolic acid and collagen derived from porcine skin. NerbridgeⓇ is a flexible, resorbable and semi-permeable tubular membrane matrix filled with porous collagen that provides a non-constricting encasement for injured peripheral nerves for protection of the neural environment. NerbridgeⓇ is designed to be an interface between the nerve and the surrounding tissue. When hydrated, NerbridgeⓇ is an easy to handle, pliable, soft, non-friable, porous conduit. The resilience of NerbridgeⓇ allows the product to recover and maintain closure without constricting the nerve once the device is placed around the nerve. NerbridgeⓇ is manufactured using validated viral inactivation and removal processes for the collagen. The product is provided sterile, non-pyrogenic, for single use only, in a variety of sizes. The outer pouch is for protection only and is not a sterile barrier. Only the contents of the inner foil pouch are sterile.
Indications for Use
NerbridgeⓇ is intended for the repair of peripheral nerve injuries in which there is no gap or where a gap closure can be achieved by flexion of the extremity.
Contraindications
Use of NerbridgeⓇ is contraindicated for anyone with a known allergy to porcine-derived materials or polyglycolic acid.
Warnings
- Do not re-sterilize.
- Do not use if the product package is damaged or opened.
- Do not use the product in cerebrospinal dura mater.
- When the implanted site is close to a joint, immobilize the limb for at least 1 week to avoid any disconnection of NerbridgeⓇ from the nerve stumps and its breakage. Based on the Japanese clinical study, no patients underwent motion range therapy within 1 week postoperatively.
- Use NerbridgeⓇ appropriately by doctors who have sufficient knowledge and experience on nerve reconstruction.
- Carefully implant NerbridgeⓇ at the location close to the joint to allow for postoperative rehabilitation. Otherwise, there may be a possibility of rearrangement and kinking of the product that may cause insufficient nerve reconstruction and joint contracture.
- Do not apply more than one NerbridgeⓇ device in a surgical procedure, because NerbridgeⓇ's effectiveness and safety have not been confirmed for multiple applications.
- The safety of early therapeutic exercise has not been confirmed when simultaneous application of the product and tendon suturing has occurred.
- In applying the product, insert both ends of nerve stump into the product at an adequate length for suturing lest a nerve end comes out of the tube.
- Because a study on effectiveness and safety of NerbridgeⓇ for a population of patients including elderly and infant patients, as well as pregnant, parturient and lactating women, has not been performed, no recommendation is made for the use of NerbridgeⓇ on such population of patients.
Precautions
- Rinse surgical gloves to remove any glove powder prior to handling NerbridgeⓇ.
- Hemostasis of the nerve stumps must be achieved prior to placement of NerbridgeⓇ. A blood clot in the lumen of NerbridgeⓇ will impede axon growth.
- Tensionless repair technique should be used to prevent tension along the length of the nerve.
- NerbridgeⓇ should be used with caution in infected regions.
Potential Product Complications
Possible device-related complications can occur with any surgical nerve repair procedure including pain, infection, open wounds such as dehiscence, neuroma, decreased or increased nerve sensitivity, hypersensitivity that may be caused by polyglycolic acid and collagen and complications associated with use of anesthesia.
Possible device defects may include protrusion, rearrangement, kinking and breakage of NerbridgeⓇ.
How Supplied
NerbridgeⓇ is supplied sterile and is intended for single use. The outer pouch is for protection only and is not a sterile barrier. Contents of the inner foil pouch are guaranteed to be sterile and nonpyrogenic unless the package is opened or damaged.
Symbols Used on Labeling

- Do not reuse after opening.

- Consult instructions for use.

- Caution: Consult instructions for use for warning and precaution information.

- Sterile unless package is opened or damaged.
Method of sterilization - ethylene oxide. 
- Do not re-sterilize.

- Temperature limits.

- Do not use if the product sterilization barrier or its packaging is compromised.

- Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.

- Catalog number

- Expiration date

- Lot number

- Manufacturer

- Inner diameter

- Length