- HOME
- THUNDERBIRDTM Next SYBR® qPCR Mix
-
THUNDERBIRDTM Next SYBR® qPCR Mix
Code No. QPX-201T, QPX-201
DESCRIPTION
-
-
THUNDERBIRD™ Next SYBR® qPCR Mix is a highly efficient 2× Master Mix for real-time PCR using SYBR® Green I. The master mix contains all required components, except primers. The master mix facilitates reaction setup, and improves the reproducibility of experiments.
This product is an improved version of THUNDERBIRD™ SYBR® qPCR Mix (Code No. QPS-201). In particular, the reaction specificity and PCR efficiency is enhanced.
-
Features
- - High specificity
The specificity for the detection of low-copy targets is improved. - - Homogeneous amplification
The dispersion of PCR efficiency between targets is reduced by a new PCR enhancer. - - Broad dynamic range
High specificity and effective amplification enable the detection of a broad dynamic range. - - Compatibility for various real-time cyclers.
The reagent is applicable to most real-time cyclers (i.e. Block type and glass capillary type). Because the passive reference dye is included with this kit, the kit can be applied to real-time cyclers that require a passive reference dye. - - Fast PCR
This reagent enables amplification using fast cycle condition. - - Utilization of dUTP
This master mix contains dUTP instead of dTTP. Therefore, the rate of false-positive detection can be reduced by adding uracil-N-glycosylase (UNG). *UNG is not supplied with this kit. Uracil-DNA Glycosylase (UNG), Heat-labile (Code No.UNG-101) can be used. - - Utilization of a visible tracking dye
This master mix contains visible tracking dye. This dye helps to eliminate pipetting errors, and does not spectrally overlap with fluorescent dyes used for qPCR and will not interfere with real-time detection.
Details
Applications
Intercalation assay with SYBR® Green I using DNA template
Storage condition
Store at -20℃, with blocking the light
Components
This kit includes the following components for 100 reactions (QPX-201T) and 500 reactions (QPX-201), with a total of 20μL per reaction. All reagents should be stored at -20°C.
| THUNDERBIRD™ Next SYBR® qPCR Mix | 1 mL |
| THUNDERBIRD™ Next SYBR® qPCR Mix | 1.67 mL x 3 |
Note:
THUNDERBIRD™ Next SYBR® qPCR Mix can be stored, protected from light, at 2-8°C for up to 3 months. For longer storage, this reagent should be kept at -20°C and protected from light. No negative effect was detected by 10 freeze-thaw cycles of THUNDERBRID™ Next SYBR® qPCR Mix.
Application Data
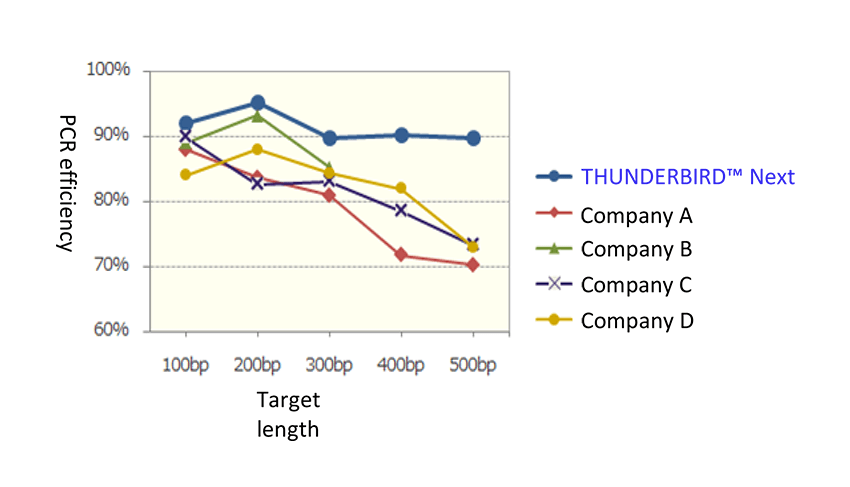
Example 1.Amplification of the 100~500bp region
Using artificial gene as template and forward primer as common, reverse primer was designed so that the amplification product lengths were 100bp, 200bp, 300bp, 400bp and 500bp. We used these primers to amplify 107 to 10 copies using THUNDERBIRD™ Next
SYBR® qPCR Mix and other companies to compare the PCR efficiency for each targets. As a result, the amplification efficiency may decrease or be undetectable as the target length increases in other companies, but stable PCR efficiency was achieved with THUNDERBIRD™ Next SYBR® qPCR Mix.
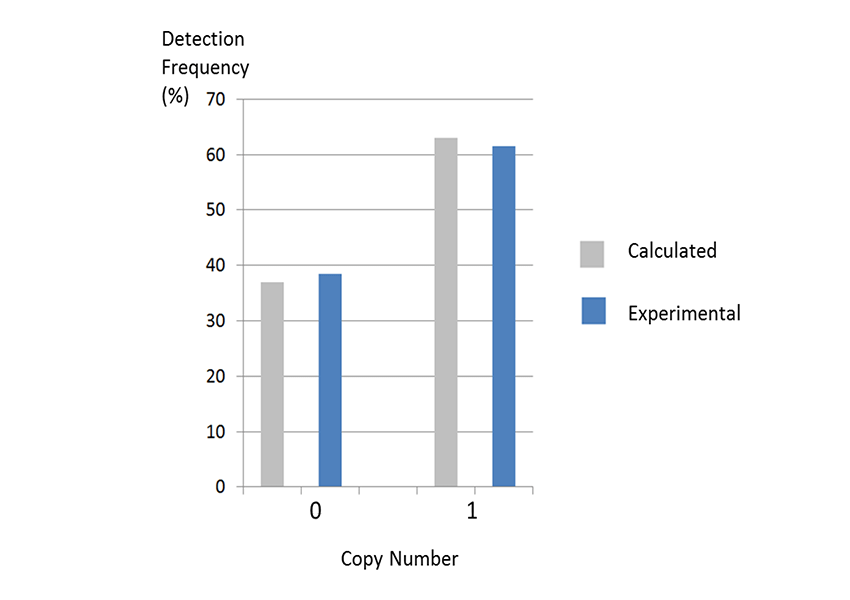
Example 2.Amplification from 1 copy gene
If one copy target can be amplified, the number of detected copies would be equivalent to the expected number of detected copies from the Poisson distribution. When one copy is added, the theoretical value from the Poisson distribution is 37% of the probability of having 0 copies and 63% of the probability of having one or more copies.
THUNDERBIRD™ Next SYBR® qPCR Mix was used to detect 96 samples using Salmonella genome diluted to one copy as a template.
The results showed that 38.5% of the samples were undetected and 61.5% were detected, which is equivalent to the expected number of samples from the Poisson distribution, suggesting that one copy equivalent can be detected.
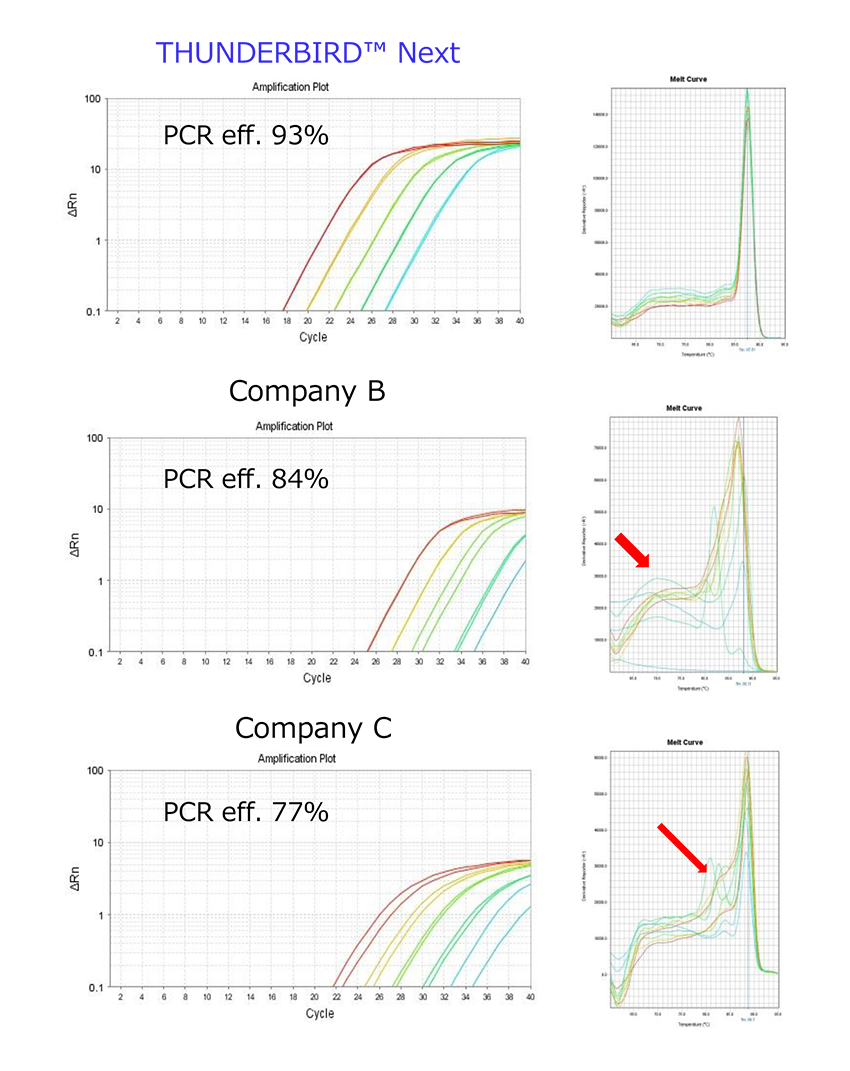
Example 3.Amplification of 65bp domain of G3PDH
cDNA from total RNA of Hela cells synthesized by reversetranscription reagents (Code No. FSQ-101) was used to amplify a 65bp G3PDH gene at 5-fold dilutions of cDNA. As a result, nonspecific amplification occurred in the low copy range in other companies, but nonspecific amplification was not observed by using THUNDERBIRD™ Next SYBR® qPCR Mix, allowing accurate quantification to the low copy range.
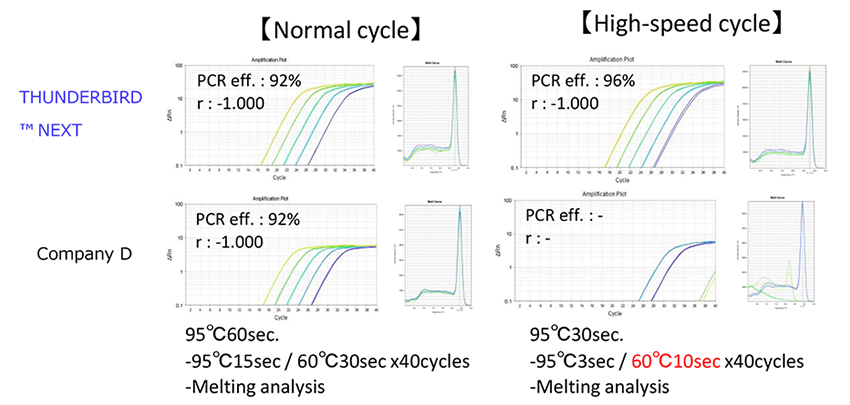
Example 4.Comparison of normal cycle and high-speed cycle
Using cDNA from Hela cells total RNA synthesized by reverse-transcription reagents (Code No. FSQ-101), amplification of β-actin gene (316bp) was amplified by "normal cycle with 30sec. extension " and "high-speed cycle with 10sec. extension". As a result, other company products that recommend normal cycling could not be amplified efficiently by high-speed cycling, but THUNDERBIRD™ Next SYBR® qPCR Mix could be amplified efficiently by high-speed cycling.
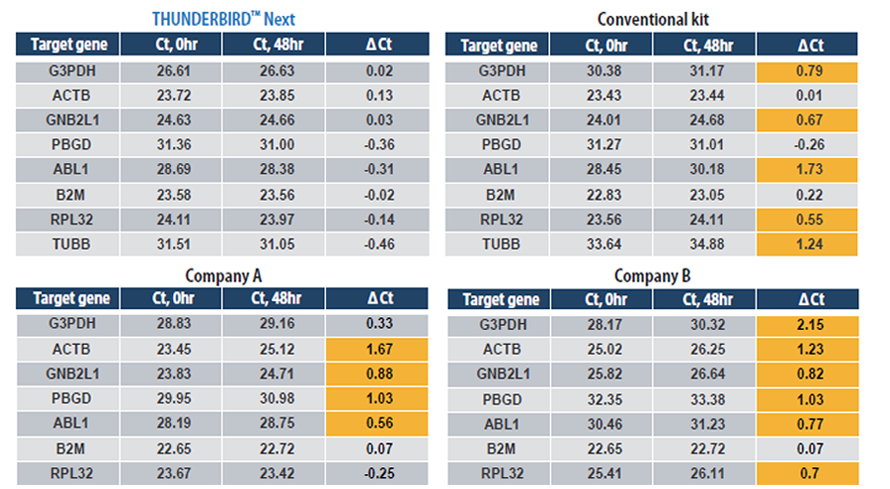
Example 5.Stability test of the prepared PCR reaction solution
Primers and template (cDNA from Hela cells total RNA) were mixed into the PCR-reaction solution, and amplifications of the targets were performed immediately or after standing for 48 hours at a light-shielding room temperature. As a result, Ct values decreased after 48 hours in our conventional kit and other products. But in THUNDERBIRD™ Next SYBR® qPCR Mix, Ct values remained stable even after 48 hours.
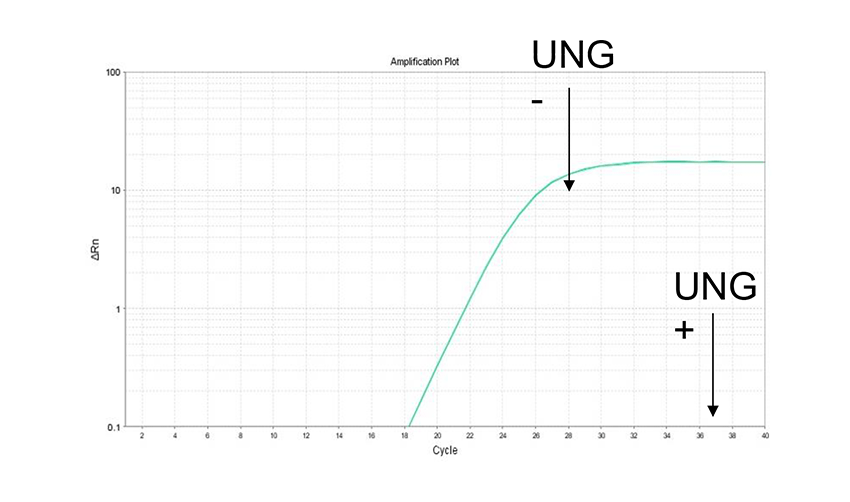
Example 6.Prevention of false positives
To confirm UNG treatment for preventing carry-over contamination. PCR products containing dUTP (104 copies) were used as template, THUNDERBIRD™ Next SYBR® qPCR Mix and Uracil-DNA Glycosylase(UNG), Heat-labile(Code No. UNG-101) were added, and amplification of the same target was performed by real-time PCR. As a result, we were able to confirm that the first PCR products were degraded by UNG treatment completely.