- HOME
- TriplucTM Luciferase Assay Reagent
-
TriplucTM Luciferase Assay Reagent
Code No. MRA-301 10mL x 1
DESCRIPTION
-
-
TriplucTM reporter assay is a powerful tool for simultaneously monitoring the expression of three genes, based on green-emitting luciferase (Stable Luciferase Green (SLG), maximum wavelength 550 nm), orange-emitting luciferase (Stable Luciferase Orange (SLO), 580 nm) and red-emitting luciferase (Stable Luciferase Red (SLR), 630 nm). TriplucTM Luciferase Assay Reagent is optimized for homogeneous assay of the three colors-emitting beetle luciferases expressed in mammalian cells.
-
Features
- ・The assay can be performed simply by adding the reagent to the cell culture and measuring luminescence after incubating for 10 minutes.
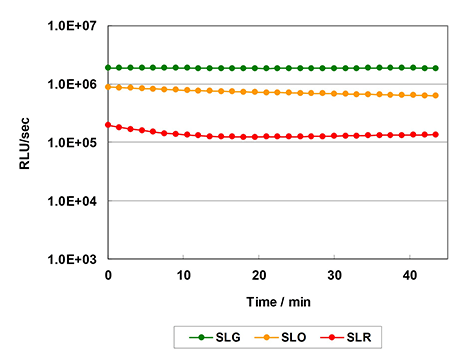
Fig. Reaction kinetics
- ・By optimizing the reagent composition to improve the luminescence duration, high-throughput measurement can be performed.
Details
Storage condition
Store at -80℃
Components
Assay reagent*
*For a 96-well plate, 10mL of Assay Reagent is equivalent to 100 reactions.
Application Data
- ・Promoter analysis
- ・Cell-based assays
- ・OECD Test Guidelines for in vitro skin sensitization test (OECD (2022), Test No. 442E)*
*This reagent is used in an international novel skin sensitization test approved by the Organization for Economic Cooperation and Development (OECD).
This test method has been validated by Tohoku University School of Medicine, Food and Drug Safety Center, Sumitomo Chemical Company, National Institute of Advanced Industrial Science and Technology (AIST) and the Japan Center for the Validation of Alternatives Methods (JaCVAM), and is useful as an alternative to animal testing in the safety evaluation of chemical substances, including cosmetic research.
REFERENCES
- Nakajima Y. et al., (2005) Multicolor luciferase assay system: one-step monitoring of multiple gene expressions with a single substrate. Biotechniques, 38, 891-894.
- Nakajima Y., Ohmiya Y. (2010) Bioluminescence assays: multicolor luciferase assay, secreted luciferase assay and imaging luciferase assay. Expert Opin. Drug Discov., 5, 835-849.
- Takahashi T. et al., (2011) An in vitro test to screen skin sensitizers using a stable THP-1-derived IL-8 reporter cell line, THP-G8. Toxicol. Sci., 124, 359-369.
- Kimura Y. et al., (2015) Optimization of the IL-8 Luc assay as an in vitro test for skin sensitization. Toxicol. In Vitro, 29, 1816-1830.
- Wakuri S. et al., (2017) Correlation between luminescence intensity and cytotoxicity in cell-based cytotoxicity assay using luciferase. Anal. Biochem., 522, 18-29.
- Kimura Y. et al., (2020) An international validation study of the IL-2 Luc assay for evaluating the potential immunotoxic effects of chemicals on T cells and a proposal for reference data for immunotoxic chemicals. Toxicol. In Vitro, 66, 104832.
- Terui H. et al., (2021) The IL-1 promoter-driven luciferase reporter cell line THP-G1b can efficiently predict skin-sensitising chemicals. Arch. Toxicol., 95, 1647-1657.
- OECD (2022), Test No. 442E: In Vitro Skin Sensitisation: In Vitro Skin Sensitisation assays addressing the Key Event on activation of dendritic cells on the Adverse Outcome Pathway for Skin Sensitisation, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris,
- Kimura Y. et al., (2023) An international validation study of the interleukin-2 luciferase leukocyte toxicity test (IL-2 Luc LTT) to evaluate potential immunosuppressive chemicals and its performance after use with the interleukin-2 luciferase assay (IL-2 Luc assay). Toxicol. In Vitro, 88, 105535.
