- HOME
- High Efficient One-step qRT-PCR Master Mix THUNDERBIRDTMNext Probe One-step qRT-PCR 4×Mix (Lyo-Ready Mix)
-
High Efficient One-step qRT-PCR Master Mix
THUNDERBIRDTMNext Probe One-step qRT-PCR 4×Mix (Lyo-Ready Mix)
Code No. QRX-101 250 reactions
DESCRIPTION
-
-
THUNDERBIRDTMNext Probe One-step qRT-PCR 4×Mix is a one-step real-time reverse-transcription polymerase chain reaction (RT-PCR) mix that uses the highly efficient reverse transcriptase “ReverTra AceTM” and Hot Start TTx DNA Polymerase. The combination of these two enzymes and our optimized buffer system enables the quickly and highly sensitively detection and quantification of small quantities of RNA. This mix does not contain glycerol and contains a lyophilization stabilizer; therefore, there should be no need to add any further excipients to assist lyophilization. After freeze-drying, it can be stored stably at room temperature and transported without the need for ice packs. In addition, real-time PCR reactions can be performed immediately by simply adding a sample containing a template and water. Freeze-drying does not impact the performance of this PCR mix.
-
Features
- Rapid and Sensitive
This corresponds to a fast cycle.
It enables rapid and sensitive detection of trace amounts of RNA.
- Easy to use
The primers, probes, and samples are simply added to initiate the reaction.
This reduces the risk of contamination and pipetting errors.
- Improving contaminant resistance
Efficient amplification can be achieved even in the presence of contaminants that inhibit PCR.
- High multiplex performance
This corresponds to the multiplex reactions that can simultaneously detect multiple genes.
- Compatibility with dUTP carryover prevention (combined with UNG sold separately)
The use of dUTP prevents false positives due to carryover contamination.
- Enables lyophilization
Excipients for lyophilization are included, allowing for the preparation of lyophilized reagents.
Details
STORAGE CONDITION
Store at -20℃
COMPONENTS
This kit includes the following components for 250 reactions (QRX-101), with a total of 20μL per reaction. All reagents should be stored at -20°C.
| THUNDERBIRD™ Next Probe One-step qRT-PCR 4×Mix | 1.25mL × 1 |
| 50× ROX reference dye | 100μL × 1 |
* Uracil-DNA Glycosylase (UNG) is not supplied with this kit.
Application Data
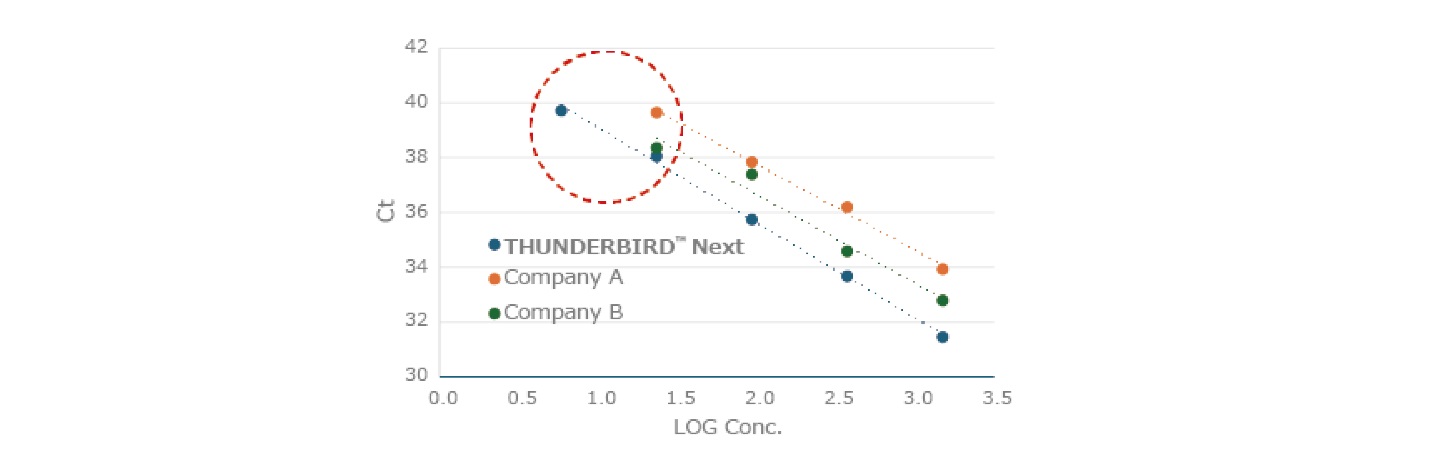
Example 1.Detection sensitivity comparison
Using TaqManTM probe, we compared the detection sensitivity and quantitation of mumps virus RNA with other companies' products.
Only this kit was able to detect RNA of less than 10 copies and quantify it over a wide dynamic range. This kit is effective in detecting RNA viruses and mRNAs with low expression levels.
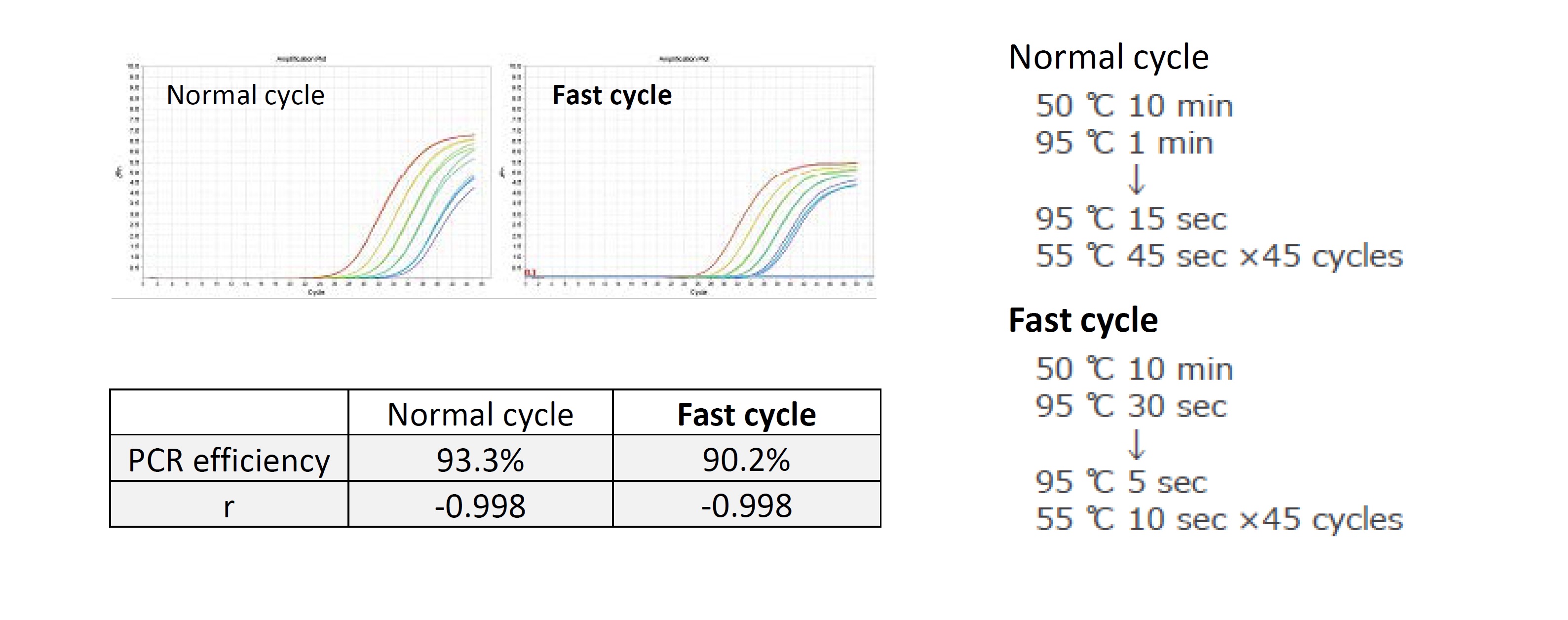
Example 2.Detection comparison during fast cycles
Influenza virus RNA was detected using the “Normal” cycle with an elongation time of 45 seconds and the “Fast” cycle with an elongation time of 10 seconds. Analysis was performed using Applied BiosystemsTM StepOnePlusTM.
As a result, efficient amplification was possible even with a fast cycle of 10 s elongation time.
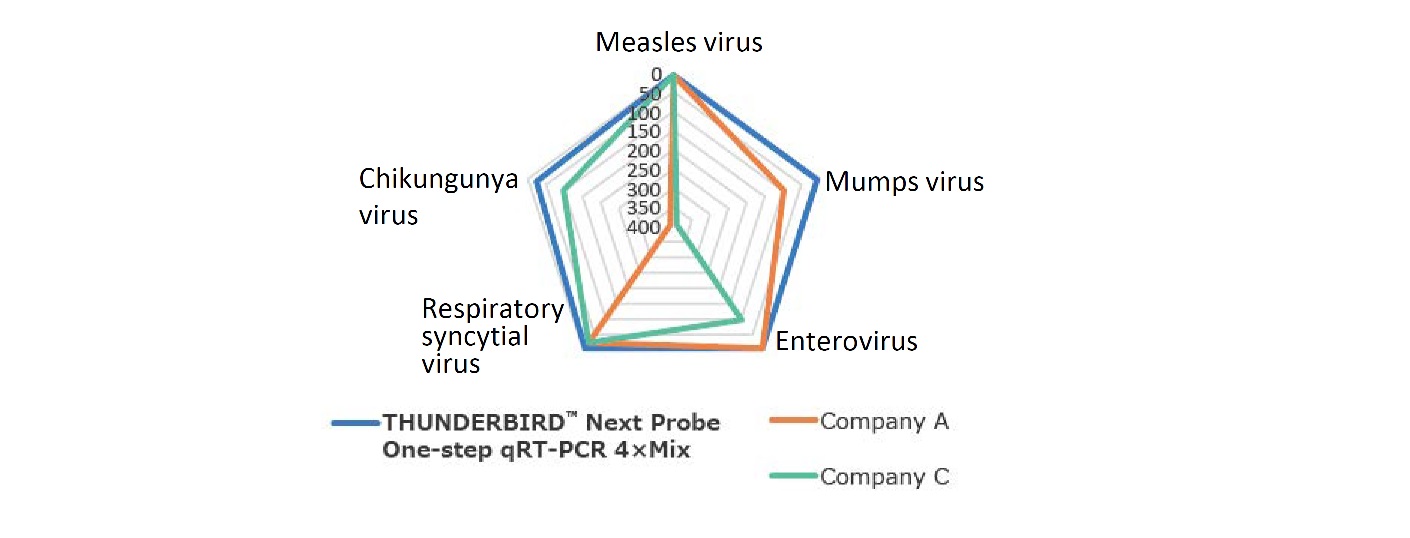
Example 3.Comparison of the detection sensitivitiesfor different viruses
The detection sensitivity of five types of viral RNA were cmpared using TaqManTM probes.
Applied BiosystemsTM StepOnePlusTM was used for the analysis.
This product was able to detect all targets with a sensitivity of 10 copies or less.
It is possible to detect various RNAs with high sensitivity, regardless of the primer/probe sequence.
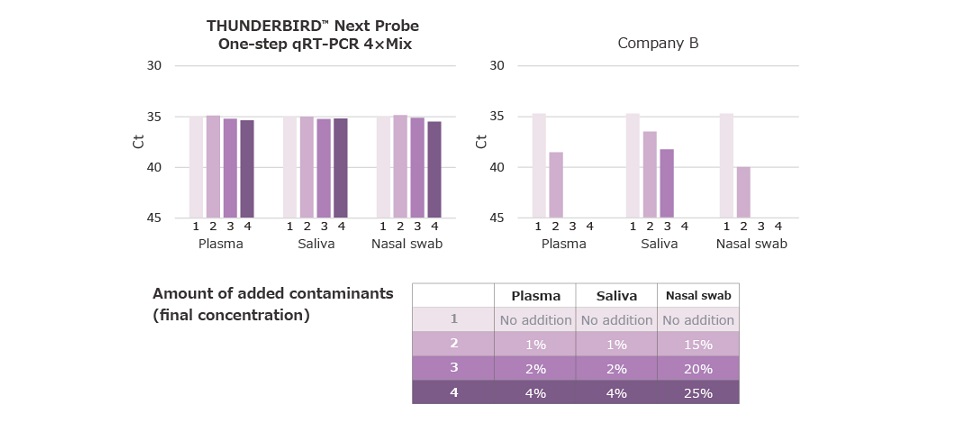
Example 4.Tolerant of contaminant
Biological samples (nasal swabs, saliva, and plasma) were added to the reaction to detect 50 copies of influenza virus RNA and evaluate resistance to PCR inhibitors. Bio-Rad CFX96 Touch Deep Well was used for the analysis.
While other company reagents caused poor amplification when PCR inhibitors were added, this reagent showed minimal effect on Ct even when biological samples were added.
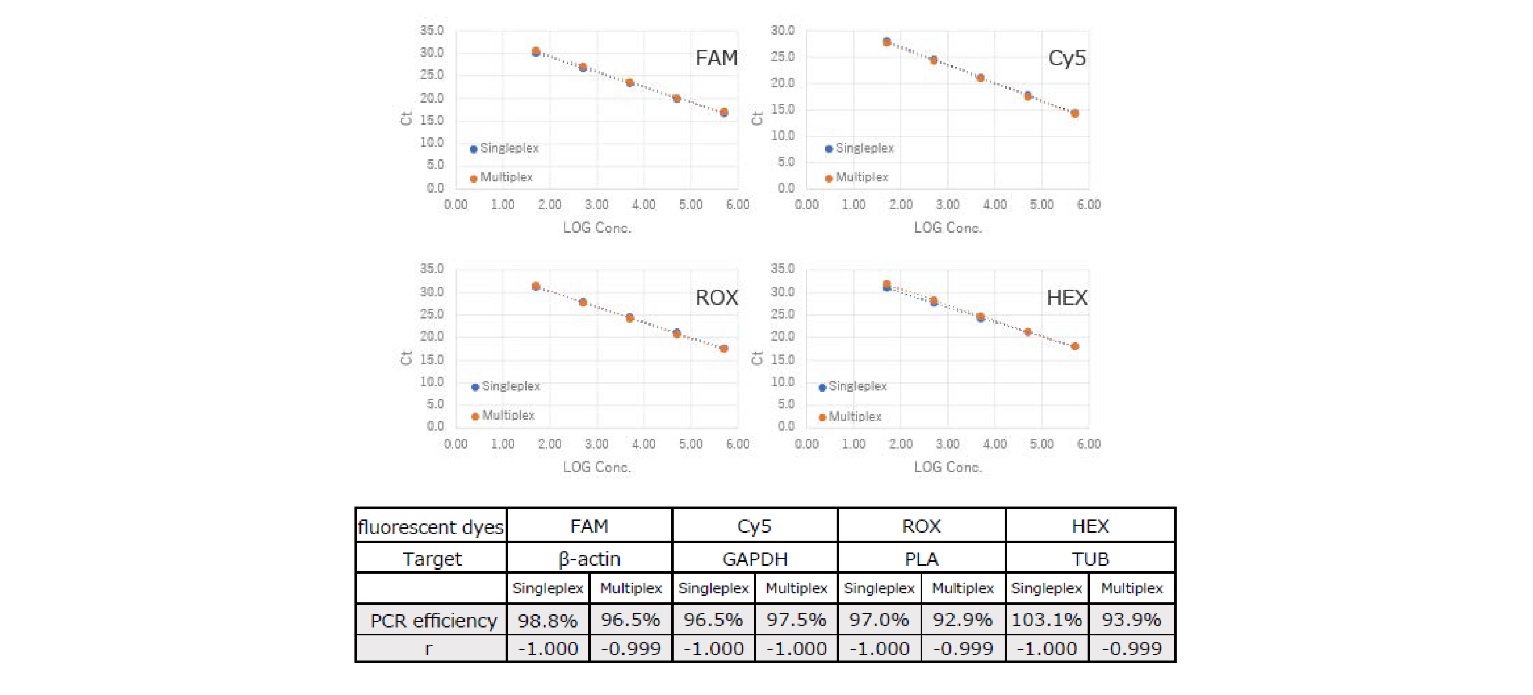
Example 5.Comparison between singleplex and multiplex methods
Single-and multiplex detections were compared using TaqManTM probes labeled with four different fluorescent dyes. HeLa S3 RNA (10x dilutions of 500 ng to 0.05 ng [5 steps]) was used as a template.
The results revealed that single and multiplex detection showed comparable PCR efficiencies and that multiplex detection did not affect the reaction.
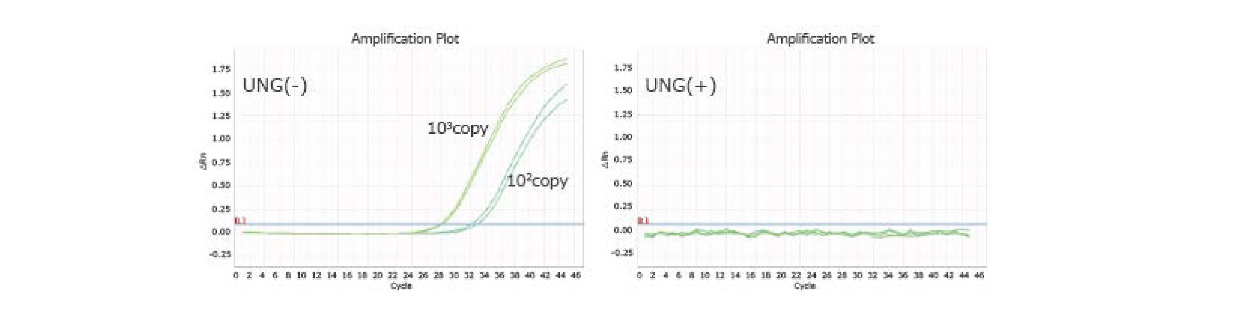
Example 6.Carryover prevention by adding UNG
Assuming that the splashed amplified product was contaminated in the subsequent PCR (carryover contamination), we used the amplified sample (196bp) equivalent to 103, 102 copies as a template and detected the same target under the conditions of UNG addition (+)/(-).
Analysis was performed using Applied BiosystemsTM StepOnePlusTM.
As a result, the template was degraded and no amplification was observed when UNG was added.
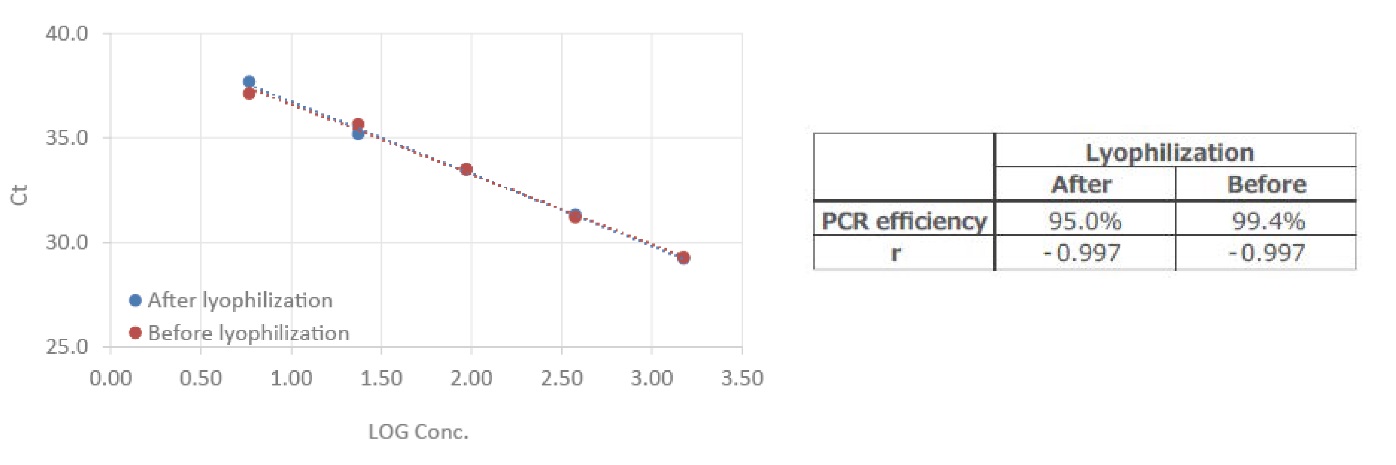
Example 7.Performance comparison before and after lyophilization
qPCR Mix was prepared using THUNDERBIRDTM Next Probe One-step qRT-PCR 4×Mix, and 1500, 375, 94, 23, and 6 copies of influenza A virus RNA were detected before and after lyophilization, respectively. Analysis was performed using a Bio-Rad CFX96 Touch Deep Well.
Good amplification was obtained in all cases and no freeze-drying effects were observed.