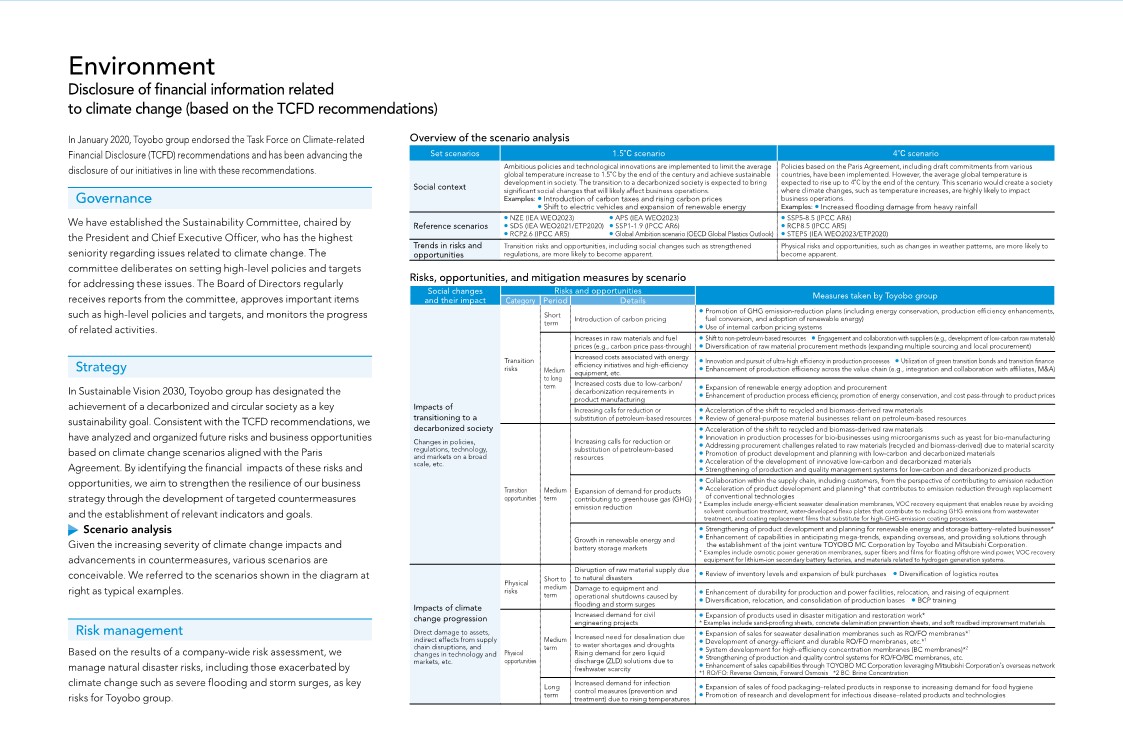
Environment
These sections introduce our group's environmental policy, environmental management structure, and related initiatives.
 A decarbonized and circular society
A decarbonized and circular society
Toyobo group recognizes climate change as a very significant social issue that will have a significant impact on our business. We support the "Glasgow Climate Pact" and aim to achieve carbon neutrality by the fiscal year 2051, which is consistent with the global 1.5℃ target.
Targets for FY2031
-
 Cutting emissions in Scope 1 & 2 by over 46% in FY2031 vs FY2014, Net zero in FY2051
Cutting emissions in Scope 1 & 2 by over 46% in FY2031 vs FY2014, Net zero in FY2051
Increasing consumption of natural resources and energy, and increasing generation of waste, are becoming serious problems throughout the world. As a result, there is a global trend to realize a transition away from conventional uni-directional resource usage toward the circular economy, in which resources are used in a sustainable manner.
Toyobo group is aiming to help realize the circular economy through its business activities by implementing initiatives to achieve the greenification (i.e., utilization of biomass and recycled raw materials, and volume reduction) of plastic in the value chain, reduce waste, and conserve water resources.
 Clean water areas, air and soil, and preservation of biodiversity
Clean water areas, air and soil, and preservation of biodiversity
Our daily lives and our economy are supported by the benefits that we receive from a wide range of living creatures and ecosystems, or in other words, from biodiversity.
Toyobo group handles a wide range of chemical products, and produces items such as textile products, containers and packaging, and raw materials for pharmaceutical products. To minimize the negative impact that our operations have on the global environment and on ecosystems, we are working to conserve biodiversity, by implementing thorough management of chemical substances, as well as striving to reduce our impact on the environment, including the atmosphere and on water area.